Sauerstoff Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
GERUCHLOSES KOMPRIMIERTES GAS
ERSCHEINUNGSBILD
VERFüSSIGTES GAS. FARBLOSE BIS BLAUE KRYOGENE FLüSSIGKEIT.
PHYSIKALISCHE GEFAHREN
Das Gas ist schwerer als Luft.
PHYSIKALISCHE GEFAHREN
Das Gas ist schwerer als Luft.
CHEMISCHE GEFAHREN
Starkes Oxidationsmittel. Reagiert mit brennbaren und reduzierenden Stoffen unter Feuer- und Explosionsgefahr.
CHEMISCHE GEFAHREN
Starkes Oxidationsmittel. Reagiert mit brennbaren und reduzierenden Stoffen unter Feuer- und Explosionsgefahr.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation.
AUFNAHMEWEGE
Aufnahme in den K?rper durch Inhalation.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Das Gas reizt die Atemwege in sehr hohen Konzentrationen. M?glich sind Auswirkungen auf Zentralnervensystem, Lunge und Augen.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Schnelle Verdampfung kann zu Erfrierungen führen. Bei sehr hohen Konzentrationen reizt die Substanz die Atemwege. M?glich sind Auswirkungen auf das Zentralnervensystem.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Risiko der Lungensch?digung bei Inhalation hoher Konzentrationen.
LECKAGE
Belüftung.
LECKAGE
Belüftung. Zündquellen entfernen. NICHT mit S?gemehl oder anderen brennbaren Absorptionsmitteln binden. Wasserstrahl NIEMALS auf die Flüssigkeit richten.
R-S?tze Betriebsanweisung:
R8:Feuergefahr bei Berührung mit brennbaren Stoffen.
R52/53:Sch?dlich für Wasserorganismen, kann in Gew?ssern l?ngerfristig sch?dliche Wirkungen haben.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S17:Von brennbaren Stoffen fernhalten.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
Beschreibung
Oxygen is a very prevalent and important element and is
necessary for sustaining life on this planet. This element is the
third most abundant in mass behind helium and hydrogen in
the universe. The diatomic form (O2) is the most common pure
form. With a boiling point at -183 °C, O2 exists as a colorless
and odorless gas at standard temperature and pressure. In the
process of cellular respiration, the highly reactive O2 is used as
the oxidant in breaking down food molecules to produce
energy. In turn, photosynthetic organisms generate O2 by using
energy from the sun and water. Other allotropes of pure oxygen
exist, including the trioxygen (O3) form known as ozone, as
well as other, less common allotropes of oxygen such as O4 and
O8. These oxygen allotropes are formed under high pressure
and low temperatures and are solid.
Chemische Eigenschaften
Oxygen, O2, is a colorless, tasteless, gaseous element essential to almost all forms of life. It promotes respiration and combustion. Oxygen comprises 20% of the earth's atmosphere and is the most abundant element in seawater and in the earth's crust. It is slightly soluble in water and alcohol, but combines readily with most other elements to form oxides. The electrolysis of water produces both oxygen and hydrogen.
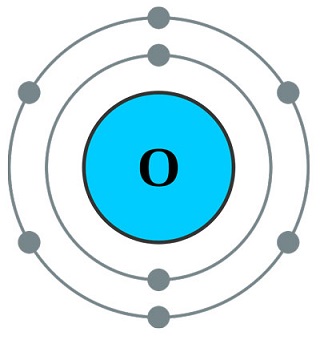
Physikalische Eigenschaften
There are three allotropes (different forms) of oxygen: (1) atomic oxygen (O), sometimesreferred to as nascent or “newborn” oxygen; (2) diatomic oxygen (O
2), or molecular oxygen(gas); and (3) ozone (O
3), also a gas.
The atmospheric oxygen that we breathe is a very reactive nonmetal and is colorless, odorless,and tasteless, but it is essential to all living organisms. It readily forms compounds withmost other elements. With six electrons in its outer valence shell, it easily gains two moreelectrons to form a negative (–2) ion; or as covalent, it can share electrons with other elementsto complete its outer shell.
Almost all the oxygen in the atmosphere (?21%) is the allotropic form of molecular oxygen(O
2). This essential gas we breathe is the result of photosynthesis, which is how green plants(with chlorophyll) use the energy of the sun to convert carbon dioxide (CO
2) and water tostarches and sugars with molecular oxygen as the by-product.
Liquid oxygen has a slightly bluish cast to it. As it boils, pure oxygen gas is released. Themelting point for oxygen is –218.79°C, its boiling point is –182.95°C, and its density is0.001429 g/cm
3.
Isotopes
There are a total of 15 isotopes of oxygen, three of which are stable. The stableones are O-16, which accounts for 99.762% of all the oxygen on Earth; O-17, whichcontributes only 0.038% of the Earth’s oxygen; and O-18, which makes up just 0.200%of Earth’s oxygen.
Charakteristisch
Oxygen is, without a doubt, the most essential element on Earth. It is required to supportall plant and animal life, and it forms more compounds with other elements than any otherelement.
Oxygen is soluble in both water and alcohol. Contrary to what many people believe, oxygenis NOT combustible (it will not burn), but rather it actively supports the combustion ofmany other substances. After all, if oxygen burned, every time a fire was lit, all the O
2 in theatmosphere would be consumed!
Burning is a form of oxidation wherein oxygen chemically combines with a substance rapidlyenough to produce adequate heat to cause fire and light, or to maintain a fire once started.The oxidation of iron is called rusting. Rusting in an example of “slow oxidation,” which isthe reaction of O
2 with Fe to form Fe
2O
3 or Fe
3O
4. This chemical reaction is so slow that theheat it produces is dissipated; thus, there is no fire.
Recently a new allotrope of oxygen was discovered. When O
2 is subjected to great pressure,it is converted intoO
4, which is a deep red solid that is a much more powerful oxidizer thanthe other forms of oxygen.
Verwenden
Oxygen has many uses due to its high electronegativity with the ability to oxidize manyother substances. Only fluorine has higher electronegativity and is thus a stronger oxidizer.Besides the essential use to support life, oxygen has many other uses.
It is used in the smelting process to free metals from their ores. It is particularly importantin the oxygen-converter process in the production of steel from iron ore.
Oxygen is used in making several important synthetic gases and in the production ofammonia, methyl alcohol, and so on.
It is the oxidizer for liquid rocket fuels, and as a gas, oxygen is used in a mixture withhelium to support the breathing of astronauts and divers and to aid patients who have difficultybreathing. It is use to treat (oxidize) sewage and industrial organic wastes.
Oxygen has many uses because of its ability to accept electrons from other elements to formionic bonds or to share electrons with other elements to form covalent bonds.
Vorbereitung Methode
Oxygen is the most prevalent element in the Earth’s crust,
making up 49.2% by weight. It accounts for 20.95% by
volume of the Earth’s atmosphere and approximately 65% by
weight of the human body.
Definition
Dioxygen: the normal form of molecularoxygen, O
2, used to distinguishit from oxygen atoms or fromozone (O
3).
Allgemeine Beschreibung
Oxygen is a colorless, odorless and tasteless gas. Oxygen will support life. Oxygen is noncombustible, but will actively support the burning of combustible materials. Some materials that will not burn in air will burn in Oxygen. Materials that burn in air will burn more vigorously in Oxygen. As a non-liquid gas Oxygen is shipped at pressures of 2000 psig or above. Pure Oxygen is nonflammable. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Oxygen is used in the production of synthesis gas from coal, for resuscitation and as an inhalant.
Reaktivit?t anzeigen
Propellant; ignites upon contact with alcohols, alkali metals, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. Heat of water will vigorously vaporize liquid Oxygen, pressures may build to dangerous levels if this occurs in a closed container. Liquid Oxygen gives a detonable mixture when combined with powdered aluminum [NFPA 491M. 1991].
Hazard
Although oxygen itself is not flammable or explosive, as is sometimes believed, its mainhazard is that, in high concentrations, oxygen can cause other materials to burn much morerapidly.
Oxygen is toxic and deadly to breathe when in a pure state at elevated pressures. In addition,such pure oxygen promotes rapid combustion and can produce devastating fires, such asthe fire that killed the Apollo 1 crew on a test launch pad in 1967. It spread rapidly because thepure oxygen was at normal pressure rather than the one-third pressure used during flight.
Oxygen used for therapeutic purposes in adults can cause convulsions if the concentrationis too high. At one time, high levels of oxygen were given to premature infants to assist theirbreathing. It was soon discovered that a high concentration of O2 caused blindness in some ofthe infants. This practice has been abandoned, or the oxygen levels have since been reduced,and this is no longer a medical problem.
Oxygen involved in metabolic processes are prone to form “free radicals,” which arethought to cause damage to cells and possibly be associated with cancer and aging.
Health Hazard
Inhalation of 100% Oxygen can cause nausea, dizziness, irritation of lungs, pulmonary edema, pneumonia, and collapse. Liquid may cause frostbite of eyes and skin.
Brandgefahr
Behavior in Fire: Increases intensity of any fire. Mixtures of liquid Oxygen and any fuel are highly explosive.
Flammability and Explosibility
Oxygen itself is nonflammable, but at concentrations greater than 25% supports and
vigorously accelerates the combustion of flammable materials. Some materials
(including metals) that are noncombustible in air will burn in the presence of oxygen.
Landwirtschaftliche Anwendung
Oxygen (O) is an odorless, colorless, gaseous element
that belongs to group 16 (formerly group VI) of the
Periodic Table. It is the most abundant element
in the earth's crust (49.2% by weight), is present in the
atmosphere (20% by volume) and is a constituent of
water. It exists in three isotopes 16, 17 and 18. Oxygen is
essential for respiration of most living organisms and for
combustion. It is used in metallurgical processes, in high
temperature flames (welding) and in medical treatment.
The common form of oxygen is di-atomic oxygen
(O
2) There is also another form - reactive allotrope
ozone (O
3)C.h emically, oxygen reacts with most other
elements forming oxides. For industrial use, it is
obtained by fractional distillation of liquid air. This has
been replaced by a process which utilizes ambient
temperature separation by means of a pressure cycle in
which molecular sieves of synthetic zeolite preferentially
absorb nitrogen from air, giving 95 % oxygen and 5 %
argon.
The most popular industrial use of oxygen is in
oxygen enrichment of steel blast furnaces. Large
quantities of oxygen are used in the synthesis of nitric
acid from ammonia, methanol and ethylene oxide, as also
in oxy-acetylene welding.
m?gliche Exposition
Compressed oxygen is used in various
oxidation processes, for feedstock; and enrichment purposes;
as a medicinal gas; a chemical intermediate; in oxyacetylene
welding; in metallurgy. Liquid oxygen is used as
a rocket fuel. Oxygen is naturally present at a concentration
of 21% in breathing air.
Carcinogenicity
Exposure to ionizing radiation is
recognized as a cause of cancer, and the production of
free radicals in the exposed cells is a part of the process.
If hyperoxia causes free radical formation, it may contribute
to the occurrence of cancer. Although a direct relation of
hyperoxic injury and cancer has not been proven, numerous
instances of association in experimental animals exist. Reactive
oxidative intermediates have been shown to cause chromosome
breaks and damage to DNA that can initiate
carcinogenesis.
Experimental work done with mouse skin tumors (as
models of human tumors) has been both revealing and
confusing. A substance may act as a cancer initiator or as
a promoter, or sometimes both, depending on intensity and
duration of exposure, and the presence of other carcinogenic
materials. The same substance can also inhibit cancer
growth. Hyperoxia has clearly been involved in modifying
the course of tumor development, but the effects have
differed under varying circumstances.
Environmental Fate
Atmospheric air contains 20.8% O2. Despite consumption of
O2 through respiration and oxidative processes, this concentration
remains constant, most likely due to the depleted O2
being replaced by plant-generated O2 in the photosynthetic
process. O2 does not bioaccumulate in organisms as pure
oxygen.
Versand/Shipping
UN1072 Oxygen, compressed & UN1073
Oxygen, refrigerated liquid (cryogenic liquid), Hazard
Class: 2.2; Labels: 2.2-Nonflammable compressed gas; 5.1-
Oxidizer. Cylinders must be transported in a secure upright
position, in a well-ventilated truck. Protect cylinder and
labels from physical damage. The owner of the compressed
gas cylinder is the only entity allowed by federal law
(49CFR) to transport and refill them. It is a violation of
transportation regulations to refill compressed gas cylinders
without the express written permission of the owner.
l?uterung methode
Purify it by passing the gas over finely divided platinum at 673oK and Cu(II) oxide (see under nitrogen) at 973o, then condensed in a liquid N2-cooled trap. HIGHLY EXPLOSIVE in contact with organic matter.
Inkompatibilit?ten
A strong oxidizer. Reacts violently with
nearly every element, combustibles, organics, and reducing
materials.
Waste disposal
Return refillable compressed
gas cylinders to supplier. Vent to atmosphere.
Sauerstoff Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Dichloracetylchlorid
4-Butylbenzoesaeure
3-(Methylthio)propanol
Osmiumtetraoxid
Rutheniumtetraoxid
Strontiumchromat
polyacrylonitrile pyrolysis product
4-Brombenzoesure
Triethylene glycol
4,4'-Bis(dimethylamino)benzophenon
2-Brombenzoesure
Tricyclo[3.3.1.13,7]decan-1-ol
Thiobencarb
3-Methylbutanon
3,5-Diisopropylphenol
4-Ethylbenzoesaeure
magnesium oxide whisker:magnesia whisker
Zirconiumtetrahydroxid
catalyst of Hopcalite MC-15���,DB-75 and DB-83
Trimangantetraoxid
Carbonylsulfid
3-Hydroxybenzoesure
Ethephon
DIETHOFENCARB
1,3-Diphenylguanidin
Micaceous Iron Oxide
4-Pentylbenzsaeure
Benzol-1,3,5-tricarbonsure
1,3-Adamantanediol
Bariumperoxid
1-Desoxy-1-(6-phenylazo-3,4-xylidino)-D-ribitol
Polyferric sulfate
Dinatriumperoxid
p-Toluyls?ure
D-ARABINONIC ACID
3,5-Dimethylbenzoesure
polypyrrole synthesized by electrolytic polymerization
Deemulsifier SP-169
Naphthalin-1,4-dicarbonsure
Carbaryl (ISO)

