HATU Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20/21/22:Gesundheitssch?dlich beim Einatmen,Verschlucken und Berührung mit der Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Beschreibung
HATU, first prepared by Louis A. Carpino in 1993, is widely used in carboxylic acid amidation reactions. It acts as a facilitator of amide bond generation by activating the carboxyl group.
Chemische Eigenschaften
White crystalline to off-white powder
Verwenden
HATU[148893-10-1] is a coupling reagent and used as an additive in peptide synthesis. It is also involved efficiently to speed up the coupling process and reduces the loss of chiral integrity.
Reagent for: Synthesis of Aurora A kinase inhibitors, HPLC assay to determine D- and L- acid enantiomers in human plasma, Amide bond formation reactions.
Catalyst for: Selective acylation, Selecocyclization-oxidation deselenation sequence.
Reaktionen
HATU is a very promising coupling agent for chemical protein synthesis.
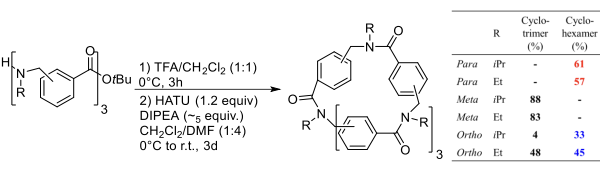
This strategy was exploited to prepare macrocycles from the trimeric linear arylopeptoids (ortho-, meta-, and para-) containing isopropyl or ethyl side chains, synthesized as described by Hjelmgaard et al. The cyclization procedure reported for α,β-cyclopeptoids was applied. The linear arylopeptoids were cyclized in the presence of HATU and DIPEA in CH?Cl?/DMF (4:1) after the deprotection of the tert-butyl group in TFA/CH?Cl?.
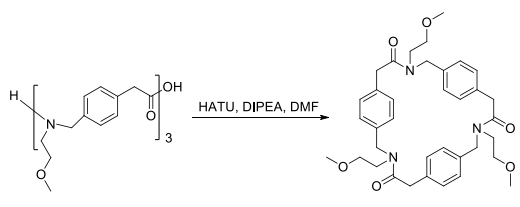
After the synthesis of the Fmoc-protected monomers, the oligomers were synthesized on the 2-chlorotrityl resin with excellent yield of coupling (>98%). The trimers and tetramers of the different isomers were synthesized in good overall yield (60-84%). Then, the crude oligomers were cyclized in DMF in the presence of HATU and DIPEA in high dilution (3 × 10?3 M) to furnish the cyclized trimers and tetramers in good yields ranging between 32% and 72%.
Synthese
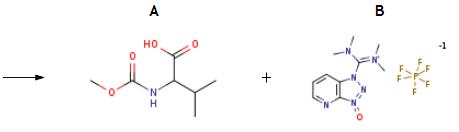
The synthesis of HATU is as follows:The resulting residue treated with 2-Methoxycarbonylamino-3-methyl-butyric acid (60 mg, 0.343 mmol) and HATU (130 mg, 0.343 mmol), suspended in DMF (3 mL) and cooled to 0° C. DIPEA (0.272 mL, 1.56 mmol) was added dropwise. After stirring for 4 h, NaOH (5M in H2O, 0.300 mL, 1.5 mmol) was added. This mixture was stirred for 3 h then diluted with EtOAc and washed with 1 M LiOH (2*) then brine. The organic phase was dried over MgSO4, filtered and concentrated. The crude residue was then purified by HPLC to afford the title compound (53 mg, 44%).
Einzelnachweise
[1] CHAWLA P A, SHOME A, JHA K T. Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium (HATU): A Unique Cross-Coupling Reagent[J]. SynOpen, 2023, 66 2: 0. DOI:
10.1055/s-0042-1751499.
[2] LOUIS A. CARPINO. Comparison of the Effects of 5- and 6-HOAt on Model Peptide Coupling Reactions Relative to the Cases for the 4- and 7-Isomers?,?[J]. Organic Letters, 2000, 2 15: 2253-2256. DOI:
10.1021/ol006013z.
HATU Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

