Levetiracetam synthesis
- Product Name:Levetiracetam
- CAS Number:102767-28-2
- Molecular formula:C8H14N2O2
- Molecular Weight:170.209
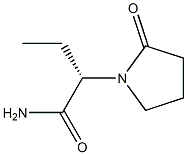
358629-51-3
21 suppliers
inquiry

102767-28-2
762 suppliers
$5.00/1g
Yield:102767-28-2 97.5%
Reaction Conditions:
with ammonium hydroxide at -5 - 0; for 8 h;
Steps:
1.D; 2.D; 3.D; 4.D D: Ammonolysis reaction
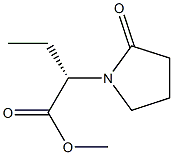
add ammonium hydroxide 60.81kg in the reactor, be cooled to -5degC , drip (S)-2-(2-oxopyrrolidin-1-yl) methyl butyrate 13.79kg, After dripping, the reaction was incubated at -50°C for 8 hours, the water was evaporated under reduced pressure, 62.5 kg of ethyl acetate was added to make pulp, centrifuged, and dried under reduced pressure to obtain 12.36 kg of the final product levetiracetam, with a molar yield of 97.5%. Starting from the starting material (S)-2-aminobutyric acid to the final product levetiracetam, the total molar yield was 48.28%, the HPLC purity was 99.90%, and the optical purity was 99.91%.
References:
CN114409586,2022,A Location in patent:Paragraph 0038; 0042-0043; 0047-0048; 0052-0053; 0057
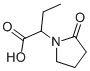
102849-49-0
170 suppliers
$95.00/100mg

102767-28-2
762 suppliers
$5.00/1g
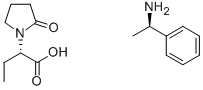
102916-46-1
30 suppliers
inquiry

102767-28-2
762 suppliers
$5.00/1g
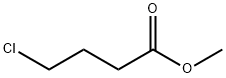
3153-37-5
256 suppliers
$13.00/5g
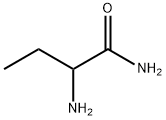
53726-14-0
70 suppliers
inquiry

102767-28-2
762 suppliers
$5.00/1g
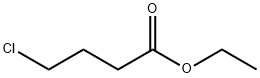
3153-36-4
172 suppliers
$14.00/25mL

53726-14-0
70 suppliers
inquiry

102767-28-2
762 suppliers
$5.00/1g