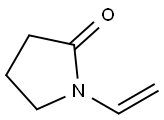
N-Vinyl-2-pyrrolidone synthesis
- Product Name:N-Vinyl-2-pyrrolidone
- CAS Number:88-12-0
- Molecular formula:C6H9NO
- Molecular Weight:111.14
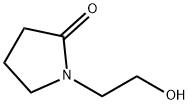
3445-11-2
146 suppliers
$7.00/5g

88-12-0
441 suppliers
$6.00/25g
Yield:88-12-0 90%
Reaction Conditions:
with Na/SiO2;ammonia at 350; under 750.075 Torr; for 10 h;Catalytic behavior;Reagent/catalyst;Temperature;
Steps:
8.B B. Preparation of N-vinylpyrrolidone
The alkaline component NH3 with N-hydroxyethylpyrrolidone steam as well as N2 were mixed. Heat to reaction temperature to obtained gaseous starting material mixture. Gaseous starting material mixture specific composition is 0.01NH3+ 0.05NHP + 0.94N2; then, The said starting material mixture, under temperature 350 °C, pressure 0.1 MPa and gas phase space velocity 2000h-1 conditons, goes through fixed bed reactor loaded with step A prepared catalyst. After reacting for 10 hours and reaching stability, collect the N-vinylpyrrolidone discharged from the fixed-bed reactor. With the embodiment 1 the same analysis method, in this embodiment the prepared N-vinyl pyrrolidone the analysis of products, obtained by the analysis result to calculate: the conversion rate of NHP 94.0%, NVP selective 95.7%, NVP yield 90.0%, recombinant sub-impurity selective 1.7%.
References:
2016 Location in patent:Paragraph 0171; 0172; 0173; 0174
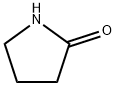
616-45-5
385 suppliers
$14.00/25g

88-12-0
441 suppliers
$6.00/25g

616-45-5
385 suppliers
$14.00/25g

111-34-2
246 suppliers
$14.00/25mL

88-12-0
441 suppliers
$6.00/25g

3445-11-2
146 suppliers
$7.00/5g

616-45-5
385 suppliers
$14.00/25g
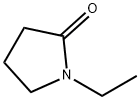
2687-91-4
456 suppliers
$6.00/25g

88-12-0
441 suppliers
$6.00/25g

616-45-5
385 suppliers
$14.00/25g

107-21-1
1339 suppliers
$10.00/25g

88-12-0
441 suppliers
$6.00/25g