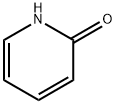
2-Hydroxypyridine synthesis
- Product Name:2-Hydroxypyridine
- CAS Number:142-08-5
- Molecular formula:C5H5NO
- Molecular Weight:95.1
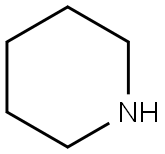
110-89-4
0 suppliers
$15.96/100ML

111-26-2
207 suppliers
$10.00/5g
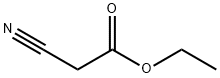
105-56-6
454 suppliers
$10.00/5ml
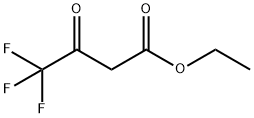
372-31-6
480 suppliers
$5.00/10g

142-08-5
513 suppliers
$6.00/5g
Yield:142-08-5 95.9%
Reaction Conditions:
with hydrogenchloride in methanol;
Steps:
1.a EXAMPLE 1
a) 100 ml of methanol and 29 g of n-hexylamine (0.287 mol) were charged initially and admixed at 30-35° C. with 32.8 g (0.287 mol) of ethyl cyanoacetate over 2 h. The mixture was subsequently stirred at 30° C. for 2 h. 25 g of piperidine were added, followed by 52.8 g (0.287 mol) of ethyl 4,4,4-trifluoroacetoacetate. The reaction mixture was then refluxed for 14 h and the solvent was removed at a temperature rising to 100° C. The rest was added while still hot to 450 g of ice and 75 ml of concentrated hydrochloric acid. The product was filtered off with suction, washed neutral with cold water and dried at 50° C. under reduced pressure, leaving 79.3 g of pyridone of formula STR5 yield: 95.9% b) 4.0 g (0.02 mol) of aldehyde of the formula STR6 and 5.8 g (0.02 mol) of the compound described in Example 1a) were heated to 130° C. in 20 ml of acetic anhydride for 30 min. Cooling brought down a precipitate of a brilliant red dye of the formula STR7 which was filtered off with suction, washed with a little methanol and dried at 50° C. under reduced pressure. Yield: 6.6 g (70%) λmax: 538 nm (CH2 Cl2)
References:
US6086637,2000,A
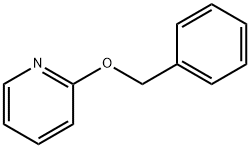
40864-08-2
34 suppliers
$45.00/50mg

142-08-5
513 suppliers
$6.00/5g
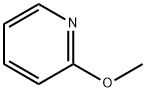
1628-89-3
341 suppliers
$17.96/25g

142-08-5
513 suppliers
$6.00/5g
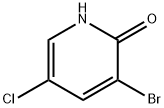
137628-16-1
125 suppliers
$9.00/250mg
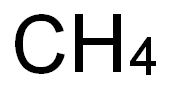
7440-44-0
317 suppliers
$12.00/25g
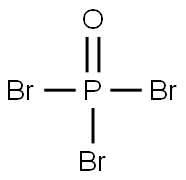
7789-59-5
224 suppliers
$10.00/5g

142-08-5
513 suppliers
$6.00/5g
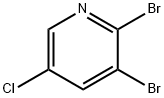
137628-17-2
250 suppliers
$8.00/5g
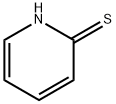
2637-34-5
316 suppliers
$6.00/5g

142-08-5
513 suppliers
$6.00/5g