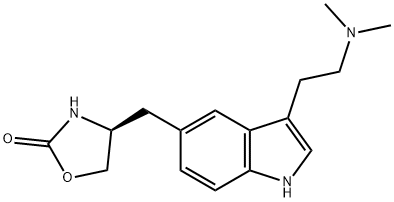
Zolmitriptan synthesis
- Product Name:Zolmitriptan
- CAS Number:139264-17-8
- Molecular formula:C16H21N3O2
- Molecular Weight:287.36
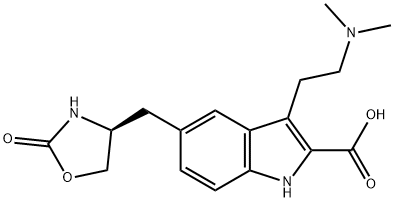
659738-69-9
20 suppliers
inquiry

139264-17-8
471 suppliers
$5.00/25mg
Yield:139264-17-8 90%
Reaction Conditions:
copper(II) oxide in quinoline at 200; for 0.333333 h;
Steps:
8 Example 8: [(S)-4- [3- (2-DIMETHYLAMINOETHYL)-IH-INDOL-5-] ylmethyl]-1, 3-oxazolidin-2-one
1 g (3.02 mmoles) of the [(S)-3- (2-] [DIMETHYLAMINOETHYL)-5- (2-OXO-1,] 3-oxazolidin-4- [YLMETHYL)-LH-INDOL-2-CARBOXYLIC] acid was suspended in 10 ml of dry quinoline. 20 mg of cuprous oxide was added and the stirred suspension heated to [200 °C] under dry nitrogen stream. The reaction mixture was kept at this temperature until no more CO2 was released (15-20 [MIN.).] It was left to cool to room temperature and the reaction mixture filtered through decalite. The filtrate was concentrated by vacuum distillation of the solvent, providing a residue which was dissolved with a succinic acid solution and washed three times with 15 ml of dichloromethane. The washed aqueous phase was cooled, the pH adjusted to 12 with a [40%] sodium hydroxide solution and extracted three times with 20 ml of dichloromethane. The combined organic phases were dried on anhydrous sodium sulphate and evaporated to dryness. The residue was recrystallised with isopropyl alcohol to give 780 mg [(90%)] of zolmitriptan as a white solid. M. p. [138-140 °C.] IS (KBr): [1237] cm-1, 1443 cm-1, 1743 cm-1. 1H-NMR (200 MHz, [CDC13)] : 2.34 (s, 6H, N (CH3)2), 2.67 (m, 2H, CH2CH2N), 2.93 (t, 4H, CH2CH2N and [CH2-BENZ.),] 4. 14 [(M,] 2H, OCH2), 4.43 (m, [1H,] NHCH) ; 5.60 (ba, [1H,] NH- [INDOLE) ;] 6.94 (dd, J=1, 2 and 8.6 Hz, 1H, ar); 7.01 (d, J=1, 2 Hz, 1H, ar) ; 7.28 (d, J=8. 6 Hz, 1H, ar), 7.37 (s, 1H, ar), 8. [49] (s, 1H, CONH). 13C-NMR [(200] MHz, CDCl3) : 23,5 ; 41,6 ; 45,3 ; 54,3 ; 60,1 ; 67, 7; 111,6 ; 113,8 ; 118,8 ; 122,4 ; 122,7 ; 126,4 ; 127,8 ; 135, 4; 159,3.
References:
WO2004/14901,2004,A1 Location in patent:Page 18-19
19718-92-4
217 suppliers
$29.00/1g
187975-62-8
32 suppliers
inquiry

139264-17-8
471 suppliers
$5.00/25mg
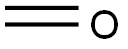
50-00-0
894 suppliers
$10.00/25g
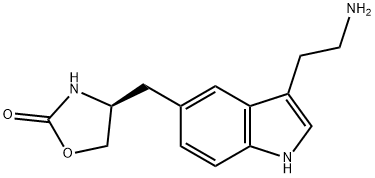
139264-15-6
46 suppliers
inquiry

139264-17-8
471 suppliers
$5.00/25mg
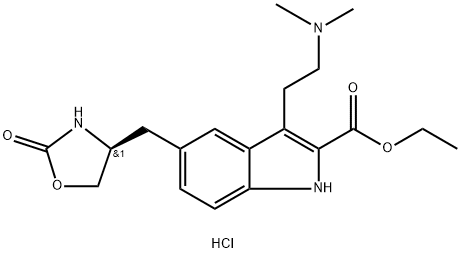
868622-23-5
8 suppliers
$165.00/10mg

139264-17-8
471 suppliers
$5.00/25mg
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
39 suppliers
inquiry

139264-17-8
471 suppliers
$5.00/25mg