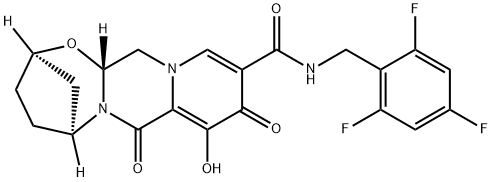
Bictegravir synthesis
- Product Name:Bictegravir
- CAS Number:1611493-60-7
- Molecular formula:C21H18F3N3O5
- Molecular Weight:449.38
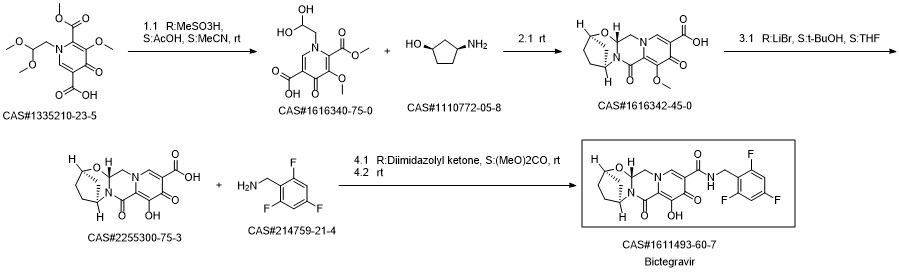
Reference: Phull, Manjinder Singh; Rao, Dharmaraj Ramachandra; Birari, Dilip Ramdas. Process for the preparation of bictegravir and intermediate thereof. Assignee Cipla Limited, India. WO 2018229798. (2018).
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-N-(2,4,6-trifluorobenzyl)-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxamide](/CAS/20180703/GIF/1616340-94-3.gif)
1616340-94-3
29 suppliers
inquiry

1611493-60-7
164 suppliers
$8.00/1mg
Yield:1611493-60-7 85%
Reaction Conditions:
with lithium bromide in tetrahydrofuran at 60;Flow reactor;
Steps:
17 Example 17: Preparation of Compound (III) from Compound (He)
A solution of (2R,5 S, l3aR )-8-methoxy -7 ,9-dioxo-N -(2,4,6-trifluorobenzyl)- 2, 3, 4, 5, 7,9, 13, l3a-octahydro-2,5-methanopyrido[r,2':4,5]pyrazino[2, l- b][l,3]oxazepine-l0-carboxamide (lie) (75g,0.237moles) in THF was then introduced in a Tube Flow Reactor and demethylated with Lithium bromide (30.98g, 0.356 moles) in THF at temperature of 60°C. After a residence time of 15 mins, the reaction mass was cooled to RT, treated with 10% Aq. HC1 solution and extracted in dichloromethane. The organic layer was concentrated & solid was isolated in isopropyl alcohol to yield Compound (III). (0330) HPLC purity > 98.0% (0331) Yield > 85%w/w
References:
WO2019/159199,2019,A1 Location in patent:Page/Page column 43; 44
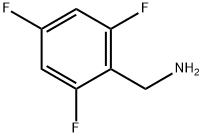
214759-21-4
215 suppliers
$9.00/250mg
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
29 suppliers
inquiry

1611493-60-7
164 suppliers
$8.00/1mg
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
29 suppliers
inquiry

1611493-60-7
164 suppliers
$8.00/1mg
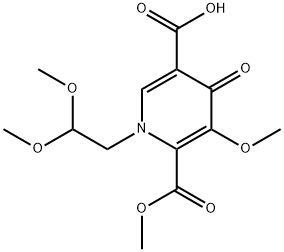
1335210-23-5
212 suppliers
$5.00/250mg

1611493-60-7
164 suppliers
$8.00/1mg

214759-21-4
215 suppliers
$9.00/250mg

1611493-60-7
164 suppliers
$8.00/1mg