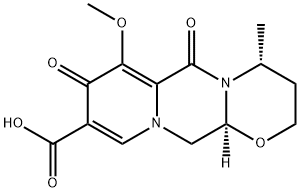
(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid synthesis
- Product Name:(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid
- CAS Number:1335210-34-8
- Molecular formula:C14H16N2O6
- Molecular Weight:308.29
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid (4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/NewsImg/2024-03-15/6384609801889233954941604.png)
61477-40-5
443 suppliers
$15.00/1g
1335210-23-5
212 suppliers
$5.00/250mg
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
69 suppliers
$1296.00/1g
Yield:1335210-34-8 65%
Reaction Conditions:
with methanesulfonic acid;glacial acetic acid in acetonitrile; for 15 h;Reflux;
Steps:
3 Example 3. Preparation of compound VIb (intermediate for synthesizing Dolutegravir)
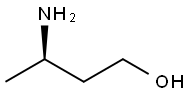
The intermediate acetal obtained in Example 1 (compound 4, 20 g, 63 mmol) was weighed and dissolved in 100 mL of CH3CN.HOAc (100 mL) and CH3SO3H (1.2 mL, 18.5 mmol) and (R)-3-amino-1-butanol (16.73 g, 188 mmol) in CH3CN (100 mL) were added at room temperature, and the mixture was refluxed for 15 hours.The mixture was concentrated, and the residue was redissolved in CH2Cl2 (200 mL).Dilute HCl (IN, 100 mL) was added and the layers were separated.The aqueous layer was extracted with CH2Cl2 (100 mL x 2), and the organic layers were combined and concentrated. MeOH (50 mL) was added and the resulting mixture was heated to reflux for 2 hours, a yellow solid precipitated from the reaction.The reaction was stopped, cooled to room temperature, and the product was collected by filtration and dried in vacuo to give the title compound VIb (12.6 g, 65% yield) as a yellow solid.
References:
CN114605437,2022,A Location in patent:Paragraph 0017; 0041-0042
41051-15-4
305 suppliers
$6.00/5g
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
69 suppliers
$1296.00/1g
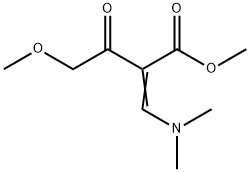
127958-23-0
10 suppliers
inquiry
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
69 suppliers
$1296.00/1g
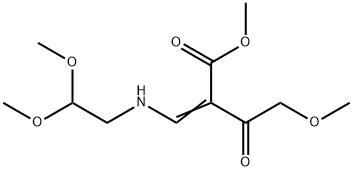
1335210-26-8
10 suppliers
inquiry
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
69 suppliers
$1296.00/1g

1335210-23-5
212 suppliers
$5.00/250mg
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
69 suppliers
$1296.00/1g