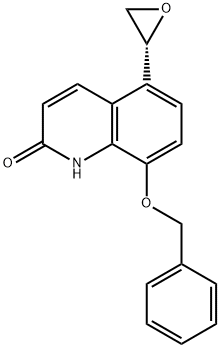
5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone synthesis
- Product Name:5-(2R)-2-Oxiranyl-8-benzyloxy-2(1H)-quinolinone
- CAS Number:173140-90-4
- Molecular formula:C18H15NO3
- Molecular Weight:293.32
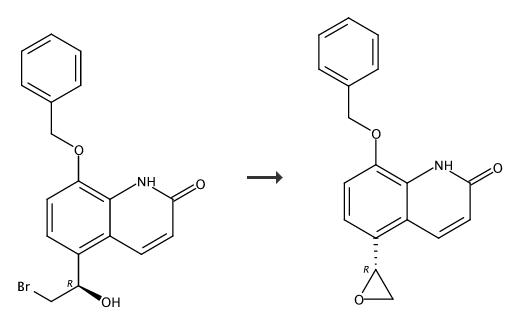
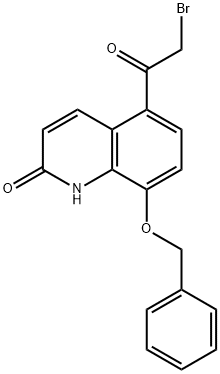
A 5 liter flask equipped with a mechanical stirrer, thermometer, and refluxing condenser was charged with 8-benzyloxy-5-[(R)-(2-bromo-1-hydroxyethyl)]-carbostyriI (70gms/0.187 moles), potassium carbonate (74 gmsl 0.536 moles), acetone (3.5 liters) and water (35 ml). The resulting slurry 'was heated to reflux and maintained for 2% hours. After completion of reaction, the hot mass was filtered on hylo bed to remove inorganics. The residue was slurried in dichloromethane (200 ml) and filtered on hyflo bed. The filtrates were combined together and concentrated under vacuum completely. The residue was dissolved. in dichloromethane (500ml) and filtered on hyflo bed to remove traces of insolubles and washed with dichloromethane(100 ml). The clear filtrate was distilled completely to obtain residue. The residue was charged with methanol (70 ml), stirred and heated to 50°C for 30 minutes. The slurry obtained was cooled to 25-30°C,chilled to 0- 5°C, stirred for 1 hour. The resulting solid was isolated by filtration, washed with methanol (30ml), followed by diisopropylether (100ml) and dried under vacuum at 60-65 °C for 10-12 hours to yield 40-41 gms of 8-benzyloxy- 5-(R)-oxiranylcarbostyril.
100331-89-3
215 suppliers
$70.00/10mg

173140-90-4
180 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
Stage #1: 8-benzyloxy-5-(2-bromoacetyl)-1H-quinolin-2-onewith dimethylsulfide borane complex;(3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole in tetrahydrofuran;toluene at 0 - 2; for 1.75 h;
Stage #2: with piperidine;water in tetrahydrofuran;toluene at 0 - 2; for 1 h;Product distribution / selectivity;
Steps:
8
Example 8Preparation of 8-benzyloxy-5-(R)-oxiranylcarbostyriI from 5-Bromoacetyl-8- benzyloxycarbostyril (compound (I); Ri = benzyl)A dry 250 ml flask equipped with a mechanical stirrer, thermometer, addition funnel and15 refluxing condenser was charged with of 5-Bromoacetyl-8-benzyloxycarbostyril (5gms/ 0.0134 moles) along with dry THF (60ml) under argon. A solution of (R)-tetrahydro-i- methyl-3,3-diphenyl-(1 H, 3H )-pyrrolo[1 ,2-c][1 ,3,2]-oxazoborolidine catalyst (2.15 ml/ 0.002 moles) in toluene was added and reaction mass was cooled to 0-20C. Then, 1 molar solution of boron dimethyl sulfide (1.62 ml/ 0.017 moles) in THF (17ml) was added in 4520 minutes while maintaining temperature of 0-2°C. The reaction was stirred further for 1 hour at same temperature and then quenched by adding solution of piperidine (8.5ml/0.0861 moles) in water (80 ml) in 30 minutes. The reaction mass was stirred at 0-2 0C for 30 minutes. After completion of reaction, reaction mass was brought to 20-22°C and extracted with dichloromethane (50ml) thrice. The combined dichloromethane extracts25 were washed with water (100 ml) thrice. The organic layer was distilled out completely under vacuum at 3O0C. To the resulting residue was added methanol (12ml), and warmed to 500C for 5-10 minutes. The slurry obtained was cooled to 25-30°Cand stirred for 30 minutes. The resulting 8-benzyloxy- 5-(R)-oxiranylcarbostyril was isolated by filtration and washed with methanol (10ml), followed by diisopropylether (30 ml), dried under vacuum at30 60-65 0C for 10-12 hours. Yield- 1.2 gms
References:
WO2008/104781,2008,A1 Location in patent:Page/Page column 40
100-39-0
439 suppliers
$10.00/10g

173140-90-4
180 suppliers
$45.00/10mg
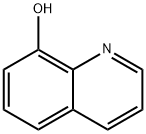
148-24-3
862 suppliers
$5.00/25g

173140-90-4
180 suppliers
$45.00/10mg
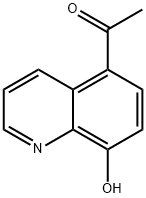
2598-31-4
57 suppliers
$160.00/250mg

173140-90-4
180 suppliers
$45.00/10mg
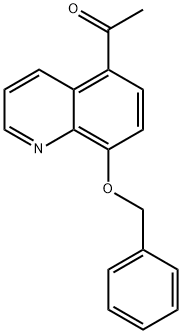
26872-48-0
13 suppliers
$150.00/25mg

173140-90-4
180 suppliers
$45.00/10mg