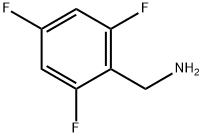
2,4,6-TRIFLUOROBENZYL AMINE synthesis
- Product Name:2,4,6-TRIFLUOROBENZYL AMINE
- CAS Number:214759-21-4
- Molecular formula:C7H6F3N
- Molecular Weight:161.12
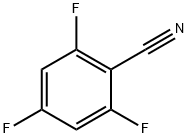
96606-37-0
241 suppliers
$6.00/1g

214759-21-4
217 suppliers
$9.00/250mg
Yield:214759-21-4 94%
Reaction Conditions:
with ammonia;hydrogen in isopropyl alcohol at 65 - 75; under 7500.75 Torr;Reagent/catalyst;Temperature;
Steps:
12 Example 12: Preparation of 2,4,6-trifluorobenzylamine
Raney Nickel (8 gm) and 2-propanol (550 gm) were charged into a reactor. The reactor was flushed once with nitrogen and then with hydrogen. The reaction mixture was heated to 65- 75°C. Hydrogen was charged in reactor at 65-75°C continuously to 10 kg/cm2. Anhydrous ammonia was charged in the reactor at 65-75°C. A solution of 2,4,6-trifluorobenzonitrile (15% in 2-propanol) was charged in the reactor using a HPLC pump at a rate of 0.5-2ml/min in 10-12 hours. After completion of the reaction, the reaction mass was cooled to 20-25°C and residual pressure was released. Reaction mass was filtered, washed with 2-propanol (200 gm). The filtrate was concentrated and purified by distillation under reduced pressure Yield (%): 94%; Purity (by GC): 97%
References:
SRF LIMITED;SINGH, Amardeep;SINGH, Bhupender;SINGH, Ram;KUMAR, Rajender;CHAUDHARY, Ajay Kumar;KUMAR, Kapil;JAIN, Anurag WO2020/152711, 2020, A1 Location in patent:Page/Page column 16-17
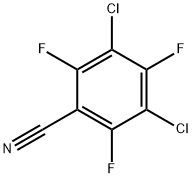
31881-89-7
8 suppliers
inquiry

214759-21-4
217 suppliers
$9.00/250mg
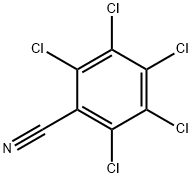
20925-85-3
189 suppliers
$6.00/1g

214759-21-4
217 suppliers
$9.00/250mg
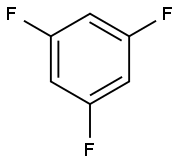
372-38-3
326 suppliers
$9.00/1g

214759-21-4
217 suppliers
$9.00/250mg
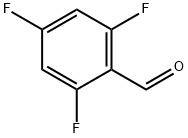
58551-83-0
201 suppliers
$8.00/1g

214759-21-4
217 suppliers
$9.00/250mg