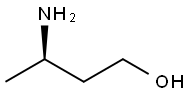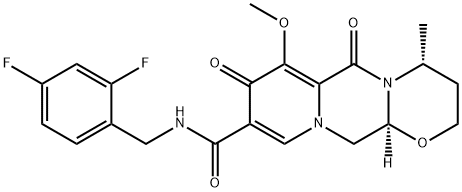
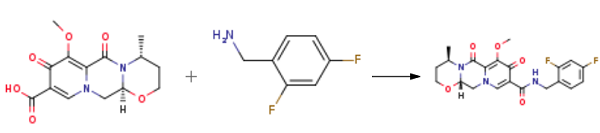
O-Methyl Dolutegravir synthesis
- Product Name:O-Methyl Dolutegravir
- CAS Number:1335210-35-9
- Molecular formula:C21H21F2N3O5
- Molecular Weight:433.41
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](/CAS/20150408/GIF/1335210-34-8.gif)
1335210-34-8
67 suppliers
$1296.00/1g
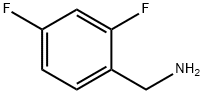
72235-52-0
404 suppliers
$12.00/5g

1335210-35-9
72 suppliers
inquiry
Yield:1335210-35-9 78%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in acetonitrile at 80; for 5 h;
Steps:
3 Example 3. Preparation of Compound 7
Compound 6 (0.6 g, 1.95 mmol),1-ethyl-(3-dimethylaminopropyl)carbonic acid diimine hydrochloride (EDCI, 0.51 g, 2.65 mmol),2,4-difluorobenzylamine (0.29 ml, 2.44 mmol) in acetonitrile (10 ml) was heated to 80 ° C for 5 h.The reaction was quenched by the addition of 6 ml of water, the lower layer was a viscous yellow oily liquid, and the upper layer was suspended.The solvent of acetonitrile was evaporated under reduced pressure and suction filtered to give a crude material (yel.The crude product was recrystallized from AcOEt/petroleum ether = 4/1 solvent 15 ml to give compound 7 0.66 g(Rf = 0.25, TLC developer: AcOEt),The yield was 78%.
References:
CN109293675,2019,A Location in patent:Paragraph 0004; 0035-0036
1335210-23-5
208 suppliers
$5.00/250mg

1335210-35-9
72 suppliers
inquiry
127958-23-0
10 suppliers
inquiry

1335210-35-9
72 suppliers
inquiry
41051-15-4
302 suppliers
$6.00/5g

1335210-35-9
72 suppliers
inquiry