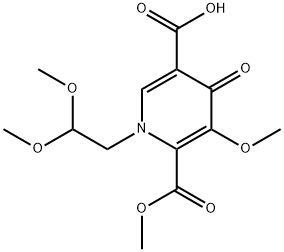
1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid synthesis
- Product Name:1-(2,2-diMethoxyethyl)-5-Methoxy-6-(Methoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid
- CAS Number:1335210-23-5
- Molecular formula:C13H17NO8
- Molecular Weight:315.28
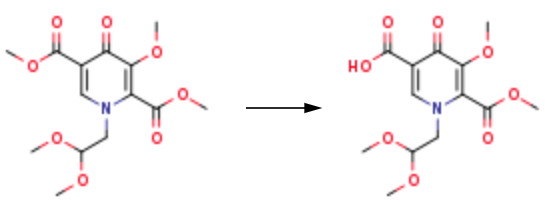
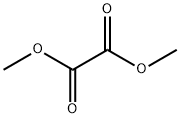
553-90-2
333 suppliers
$10.00/5g
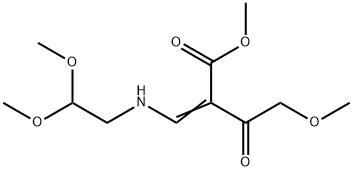
1335210-26-8
9 suppliers
inquiry

1335210-23-5
222 suppliers
$5.00/250mg
Yield:-
Reaction Conditions:
Stage #1: Dimethyl oxalate;methyl 2-(((2,2-dimethoxyethyl)amino)methylene)-4-methoxy-3-oxobutanoatewith lithium hydride in methanol at -25 - 40; for 14 h;
Stage #2: with methanol;lithium hydroxide at -5 - 5; for 1 h;
Stage #3: with hydrogenchloride in methanol;water at -5;
Steps:
A
A. l-[2,2-Bis(methyloxy)ethyl]-5-(methyloxy)-6-[(methyIoxy)carbonyl]-4-oxo-l,4-dihydro- 3-pyridinecarboxylic acidA mixture of methyl 4-methoxyacetoacetate (20 mL) and DMFDMA (24 mL) was stirred at room temperature for 1.5 h. The reaction mixture was diluted with MeOH (50 mL) and aminoacetaldehyde dimethyl acetal (16.7 mL) was added. The mixture was stirred for 1 h at room temperature, concentrated, and then diluted with MeOH (113 mL). Dimethyl oxalate (45.66 g) was charged followed by portion-wise addition of LiH (2.15 g) while maintaining the reaction temperature below 25 °C. The reaction content was heated to 40 °C for 14 h. The reaction mixture was cooled to -5 °C and LiOH (14.82 g) was added while maintaining the reaction temperature below 5 °C. When addition was complete, the mixture was stirred for a further 2 h at 3-5 °C for 1 h. The reaction mixture was quenched with aqueous HC1 (2 N, 367 mL), maintaining the reaction temperature below 5 °C. When addition was complete, EtOAc (450 mL) was added and the mixture was warmed to 20 °C. The reaction mixture was filtered and the aqueous layer discarded. Water (225 mL) was added and the organic layer was removed under reduced pressure. The product was collected by filtration and dried in a vacuum oven overnight at 50 °C. The product was obtained as a solid.
References:
WO2011/119566,2011,A1 Location in patent:Page/Page column 8

1335210-26-8
9 suppliers
inquiry

1335210-23-5
222 suppliers
$5.00/250mg
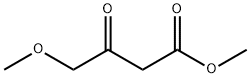
41051-15-4
311 suppliers
$6.00/5g

1335210-23-5
222 suppliers
$5.00/250mg
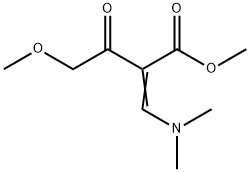
127958-23-0
11 suppliers
inquiry

1335210-23-5
222 suppliers
$5.00/250mg
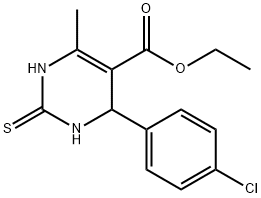
154866-92-9
6 suppliers
inquiry

1335210-23-5
222 suppliers
$5.00/250mg