Sildenafil Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S22:Staub nicht einatmen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Beschreibung
Sildenafil was launched as Viagra in the US for the treatment of organic orland psychological male erectile dysfunction (ED). It is an orally bioavailable pyrazolopyrimidinone derivative structurally related to zaprinast, with vasodilating and potential anti-inflammatory activities. Upon oral administration, sildenafil selectively targets and inhibits cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), thereby inhibiting the PDE5-mediated degradation of cGMP found in smooth muscle and increasing cGMP availability. This results in prolonged smooth muscle relaxation in the corpus cavernosum of the penis, thereby causing vasodilation, blood engorgement and a prolonged penile erection.
Chemische Eigenschaften
Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Solubility of sildenafil citrate is pH dependent and it decreases with increase of pH. pH ranges between 3.7 and 3.8 and the pKa from 8.2 to 9.6.
Verwenden
Sildenafil is a phosphodiesterase-5 (PDE5) inhibitor. It is indicated for the treatment of erectile dysfunction (ED). Sildenafil is an orally active selective type 5 cGMP phosphodiesterase inhibitor.
synthetische
The first synthetic route of sildenafil accomplished the preparation of its pyrazole derivative from ethyl 3-butyrylpyruvate and hydrazine hydrate in acetic acid, followed by the selective N-methylation of the pyrazole ring with dimethyl sulfate. The carboxylic acid was obtained after alkaline hydrolysis was subjected to nitration, followed by treatment with concentrated ammonium hydroxide solution to sequentially deliver the corresponding carboxamide derivative. The nitro group of the mentioned carboxamide derivative was then reduced to an amino group by stannous chloride/hydrochloric acid in ethanol, leading to the formation of the main 4-aminopyrazole structure. Mild amidation of the aminopyrazole derivative by the appropriate benzoyl chloride was performed, followed by cyclization mediated by hydrogen peroxide under basic environment which led to the formation of pyrimidinone heterocycle ring. Chloro-suIphonylation of pyrimidinone derivative imposed selectively on the 50 position of the phenyl ring, led to the aroyl sulfonyl chloride derivative which was then coupled with N-methylpiperazine to afford sildenafil.
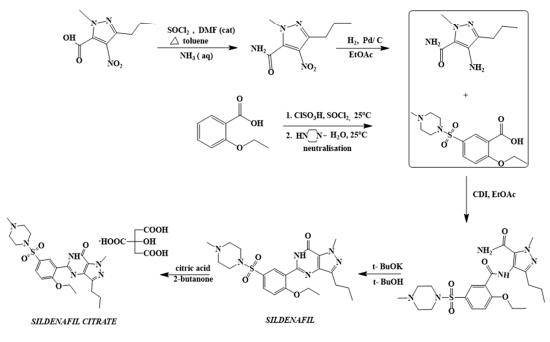 synthesis of sildenafil
synthesis of sildenafil
Definition
ChEBI: Sildenafil is a pyrazolo[4,3-d]pyrimidin-7-one having a methyl substituent at the 1-position, a propyl substituent at the 3-position and a 2-ethoxy-5-[(4-methylpiperazin-1-yl)sulfonyl]phenyl group at the 5-position. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It is a pyrazolopyrimidine, a member of piperazines and a sulfonamide.
Indications
Sildenafil (Viagra) is a selective
inhibitor of PD-5, an enzyme that inactivates cGMP.
Vardenifil (Levitra) is a particularly effective inhibitor
of PD-5. It has a shorter onset of action and can be used
in smaller doses than sildenafil. Other drugs used in the
treatment of ED exert their effects through other biochemical
pathways, both central and peripheral.
Mechanism of action
Sildenafil is readily absorbed after oral administration
and reaches peak plasma levels after about an
hour. It undergoes hepatic metabolism and has a terminal
half-life of about 4 hours.An initial dose of 50 mg is
taken about an hour prior to sexual activity to induce
penile erection.
Clinical Use
Sildenafil is a selective inhibitor of cGMP-specific
PD-5 and therefore inhibits the degradation of cGMP.
PD-5, the predominant type in the corpus cavernosum,
also is present in other tissues (e.g., lungs, platelets, and
eye). The selective inhibition of this enzyme facilitates
the release of nitric oxide and smooth muscle relaxation
of the corpus cavernosa. Sildenafil enhances erection by
augmenting nitric oxide–mediated relaxation pathways.
It has been suggested that sildenafil’s mechanism of
action is due to cross-talk between cGMP- and cAMPdependent
transduction pathways within the cavernous
muscles.
Nebenwirkungen
Orally administered sildenafil is an effective and
well-tolerated treatment for men with ED, including
those with diabetes mellitus. It has also been used for
so-called salvage therapy in men who do not respond to
intracorporeal injections of other agents.
Headache is a common side effect, as are flushing
and rhinitis.More serious side effects include definite or
suspected myocardial infarctions and cardiac arrest.
Enzyminhibitor
Sildenafil is rapidly absorbed and peaks in concentration (127–560 ng/mL) after 0.5 to 2.0 hours, displaying a half-life of 3 to 4 hours
for the full therapeutic dose (25–100 mg). It is 96% bound to plasma proteins and is metabolized by the liver CYP3A4. The
metabolite N-desmethylsildenafil possesses approximately 50% of the activity of the parent molecule.
Stoffwechsel
In vitro metabolism studies for sildenafil have shown that the primary metabolite, N-desmethylsildenafil, and the minor metabolite, oxidative opening of the piperazine ring, are mediated by CYP3A4, CYP2C9, CYP2C19, and CYP2D6. The estimated relative contributions to clearance were 79% for CYP3A4, 20% for CYP2C9, and less than 2% for CYP2C19 and CYP2D6. These results demonstrate that CYP3A4 is the primary cytochrome mediating N-demethylation and that drugs inhibiting CYP3A4 likely impair sildenafil biotransformation and clearance. The pharmacokinetics of radiolabeled sildenafil were consistent with rapid absorption, first-pass metabolism, and primarily fecal elimination of N-demethylated metabolites. The absorption of sildenafil following oral administration was rapid (~92%), whereas the oral bioavailability was approximately 38% as a result of first-pass metabolism.
Sildenafil Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

