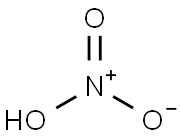
Salpetersure
|
|
Salpetersure Eigenschaften
- Schmelzpunkt:
- -42 °C
- Siedepunkt:
- 120.5 °C(lit.)
- Dichte
- 1.41 g/mL at 20 °C
- Dampfdichte
- 1 (vs air)
- Dampfdruck
- 8 mm Hg ( 20 °C)
- Flammpunkt:
- 120.5°C
- storage temp.
- Store at +2°C to +25°C.
- L?slichkeit
- Miscible with water.
- pka
- -1.3(at 25℃)
- Aggregatzustand
- Liquid, Double Sub-Boiling Quartz Distillation
- Wichte
- d 20/4 1.4826
- Farbe
- colorless to deep yellow
- S?ure-Base-Indikators(pH-Indikatoren)
- 1
- Geruch (Odor)
- Suffocating fumes detectable at <5.0 ppm
- PH
- 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution);
- Wasserl?slichkeit
- >100 g/100 mL (20 ºC)
- Sensitive
- Hygroscopic
- Merck
- 14,6577
- Expositionsgrenzwerte
- TLV-TWA 2 ppm (5 mg/m3) (ACGIH, MSHA, OSHA, and NIOSH); STEL 4 ppm (~10 mg/m3) (ACGIH).
- Dielectric constant
- 55.0(14℃)
- LogP
- -0.773 (est)
- CAS Datenbank
- 7697-37-2(CAS DataBase Reference)
- NIST chemische Informationen
- Nitric acid(7697-37-2)
- EPA chemische Informationen
- Nitric acid (7697-37-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | C,O,Xi,T+ | ||
|---|---|---|---|
| R-S?tze: | 8-35-34-20-41-37/38-36/38-26/27 | ||
| S-S?tze: | 23-26-36-45-36/37/39-39-60-28 | ||
| RIDADR | UN 3264 8/PG 3 | ||
| OEB | B | ||
| OEL | TWA: 2 ppm (5 mg/m3), STEL: 4 ppm (10 mg/m3) | ||
| WGK Germany | 1 | ||
| RTECS-Nr. | QU5900000 | ||
| F | 8 | ||
| TSCA | Yes | ||
| HS Code | 2808 00 00 | ||
| HazardClass | 8 | ||
| PackingGroup | II | ||
| Giftige Stoffe Daten | 7697-37-2(Hazardous Substances Data) | ||
| Toxizit?t | LC50 inhal (rat) 2500 ppm (1 h) PEL (OSHA) 2 ppm (5 mg/m3) TLV-TWA (ACGIH) 2 ppm (5.2 mg/m3) STEL (ACGIH) 4 ppm (10 mg/m3) |
||
| IDLA | 25 ppm |
| Bildanzeige (GHS) |
  
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Salpetersure Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS GELBE FLüSSIGKEIT MIT STECHENDEM GERUCH.CHEMISCHE GEFAHREN
Zersetzung beim Erw?rmen unter Bildung von Stickoxiden. Starkes Oxidationsmittel. Reagiert heftig mit brennbaren und reduzierenden Stoffen, z.B. Terpentin, Aktivkohle, Alkohol.. Starke S?ure. Reagiert sehr heftig mit Basen. ?tzend gegenüber Metallen unter Bildung brennbarer/explosionsf?higer Gase (z.B. Wasserstoff, ICSC-Nr. 0001).ARBEITSPLATZGRENZWERTE
TLV: 2 ppm (als TWA), 4 ppm (als STEL); (ACGIH 2006).MAK: IIb (nicht festgelegt, aber Informationen vorhanden) (DFG 2008).
AUFNAHMEWEGE
Schwerwiegende lokale Wirkungen auf allen Aufnahmewegen.INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C kann sehr schnell eine gesundheitssch?dliche Kontamination der Luft eintreten.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Die Substanz ver?tzt die Augen, die Haut und die Atemwege. ?tzend beim Verschlucken. Inhalation kann zu Lungen?dem führen (siehe ANMERKUNGEN). Die Auswirkungen treten u.U. verz?gert ein (siehe ANMERKUNGEN).
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Risiko der Lungensch?digung bei wiederholter oder l?ngerer Dampf-Exposition. M?glich sind Auswirkungen auf die Z?hne mit nachfolgender Zahnerosion.LECKAGE
Gefahrenbereich verlassen! Fachmann zu Rate ziehen! Pers?nliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabh?ngigem Atemschutzger?t. Belüftung. Ausgelaufene Flüssigkeit in abdichtbaren Beh?ltern sammeln. Reste vorsichtig mit Natriumcarbonat neutralisieren. Dann mit viel Wasser wegspülen. NICHT mit S?gemehl oder anderen brennbaren Absorptionsmitteln binden.R-S?tze Betriebsanweisung:
R8:Feuergefahr bei Berührung mit brennbaren Stoffen.R35:Verursacht schwere Ver?tzungen.
R34:Verursacht Ver?tzungen.
R20:Gesundheitssch?dlich beim Einatmen.
S-S?tze Betriebsanweisung:
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
Nitric acid is a colorless, corrosive liquid that is the most common nitrogen acid. It has been used for hundreds of years. Nitric acid is a mineral acid that was called spirit of nitre and aqua fortis, which means strong water.
Fuming nitric acid is named because of the fumes emitted by acid when it combines with moist air. Fuming nitric acid is highly concentrated and is labeled either red fuming nitric acid or white fuming nitric acid. Red fuming nitric acid, as the name implies, emits a reddishbrown fume on exposure to air. The color comes from nitrogen dioxide, which is liberated on exposure to air. The nitric acid concentration of red fuming nitric acid is approximately 85% or greater, with a substantial amount of dissolved nitrogen dioxide. White fuming nitric acid is highly concentrated anhydrous nitric acid with concentrations of 98–99%; the remaining 1–2% is water and nitrogen dioxide. Most commercial grade nitric acid has a concentration of between 50% and 70%.
Chemische Eigenschaften
Nitricacid,HN03, is a strong,fire-hazardous oxidant. It is a colorless or yellowish liquid that is miscible with water and boils at 86℃ (187 ℉). Nitric acid, also known as aqua fortis, is used for chemical synthesis, explosives, and fertilizer manufacture, and in metallurgy, etching, engraving, and ore flotation.Physikalische Eigenschaften
Colorless liquid; highly corrosive; refractive index 1.397 at 16.5°C; density 1.503 g/L; freezes at –42°C; boils at 83°C; completely miscible with water; forms a constant boiling azeotrope with water at 68.8 wt% nitric acid; the azeotrope has density 1.41 g/mL and boils at 121°C.Verwenden
Nitric acid is an important starting material for the production of fertilizers and chemicals. Diluted nitric acid is used for dissolving and etching metals Product Data SheetDefinition
nitric acid: A colourless corrosivepoisonous liquid, HNO3; r.d. 1.50;m.p. –42°C; b.p. 83°C. Nitric acid maybe prepared in the laboratory by thedistillation of a mixture of an alkalimetalnitrate and concentratedsulphuric acid. The industrial productionis by the oxidation of ammoniato nitrogen monoxide, theoxidation of this to nitrogen dioxide,and the reaction of nitrogen dioxidewith water to form nitric acid and nitrogenmonoxide (which is recycled).The first reaction (NH3 to NO) iscatalysed by platinum or platinum/rhodium in the form of fine wiregauze. The oxidation of NO and theabsorption of NO2 to form the productare noncatalytic and proceedwith high yields but both reactionsare second-order and slow. Increasesin pressure reduce the selectivity ofthe reaction and therefore ratherlarge gas absorption towers are required.In practice the absorbing acidis refrigerated to around 2°C and acommercial ‘concentrated nitric acid’at about 67% is produced.Nitric acid is a strong acid (highlydissociated in aqueous solution) anddilute solutions behave much likeother mineral acids. Concentrated niniobium tric acid is a strong oxidizing agent.Most metals dissolve to form nitratesbut with the evolution of nitrogenoxides. Concentrated nitric acid alsoreacts with several nonmetals to givethe oxo acid or oxide. Nitric acid isgenerally stored in dark brown bottlesbecause of the photolytic decompositionto dinitrogen tetroxide. Seealso nitration.
Allgemeine Beschreibung
Nitric acid is a colorless to yellow or red liquid sometimes fuming reddish brown vapors with a suffocating odor. Nitric acid is soluble in water with release of heat. Nitric acid is corrosive to metals or tissue. Nitric acid will accelerate the burning of combustible materials and Nitric acid may even cause ignition upon contact with combustible material. Nitric acid is fully soluble in water and may react violently upon contact with water with the evolution of heat, fumes and spattering. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Density 10.4 lb / gal.Air & Water Reaktionen
Fumes in air. Fully soluble in water with release of heat. Reacts violently with water with the production of heat, fumes, and spattering.Reaktivit?t anzeigen
Nitric acid; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. The reaction of finely divided antimony and nitric acid can be violent [Pascal 10:504. 1931-34]. Bromine pentafluoride reacts violently with strong nitric acid and strong sulfuric acid [Mellor 2, Supp. 1:172. 1956]. Experiments show that mixtures of over 50% nitric acid by weight in acetic anhydride may act as detonating explosives [BCISC 42:2. 1971]. An etching agent of equal portions of acetone, nitric acid, and 75% acetic acid exploded 4 hours after Nitric acid was prepared and placed in a closed bottle. This is similar to a formulation for the preparation of tetranitromethane a sensitive explosive [Chem. Eng. News 38: 56. 1960]. Phosphine is violently decomposed by concentrated nitric acid, and flame is produced. Warm fuming nitric acid, dropped in a container of phosphine gas produces an explosion [Edin. Roy. Soc. 13:88. 1835]. An explosion occurs when nitric acid is brought into contact with phosphorus trichloride [Comp. Rend. 28:86]. The reaction of sodium azide and strong nitric acid is energetic [Mellor 8, Supp 2:315. 1967]. Reacts violently with water with the production of heat, fumes, and spattering.Hazard
Because it is a strong oxidizing agent, nitric acid may undergo violent reactions with powerful reducing agents. Many nitration reactions of organics yield explosive products. Pure nitric acid is highly corrosive to skin causing severe injury. Concentrated acid (68.8 wt %) is moderately corrosive to skin. The acid may decompose under heating or photochemically, liberating toxic nitrogen dioxide gas.Health Hazard
Nitric acid is a corrosive substance causingyellow burns on the skin. It corrodes the bodytissues by converting the complex proteinsto a yellow substance called xanthoproteicacid (Meyer 1989). Ingestion of acid canproduce burning and corrosion of the mouthand stomach. A dose of 5–10 mL can befatal to humans.Chronic exposure to the vapor and mist ofnitric acid may produce bronchitis and chemical pneumonitis (Fairhall 1957). It emitsNO2, a highly toxic gas formed by its decomposition in the presence of light. Nitric acidis less corrosive than sulfuric acid. Its vaporand mist may erode teeth..
Brandgefahr
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Flammability and Explosibility
Explosibility Not a combustible substance, but a strong oxidizer. Contact with easily oxidizible materials including many organic substances may result in fires or explosions.Industrielle Verwendung
Also called aqua fortis and azotic acid, nitricacid is a colorless to reddish fuming liquid ofthe composition HNO3, having a wide varietyof uses for pickling metals, etching, and in themanufacture of nitrocellulose, plastics, dyestuffs,and explosives. It has a specific gravityof 1.502 (95% acid) and a boiling point of 86°C,and is soluble in water. Its fumes have a suffocatingaction, and it is highly corrosive andcaustic. Fuming nitric acid is any water solutioncontaining more than 86% acid and having aspecific gravity above 1.480. Nitric acid is madeby the action of sulfuric acid on sodium nitrateand condensation of the fumes. It is also madefrom ammonia by catalytic oxidation, or fromthe nitric oxide produced from air.Sicherheitsprofil
Poison by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. A very dangerous fire hazard and very powerful oxidizing agent. Can react explosively with many reducing agents. Wdl react with water or steam to produce heat and toxic, corrosive, and flammable vapors.When heated to decomposition it emits hghly toxic fumes of NOx. See also NITRIC ACID.Sicherheit(Safety)
Nitric acid is used in the manufacture of ammonium nitrate fertilizer and explosives, in steel etching, and in reprocessing spent nuclear fuel. There are two types of fuming nitric acid. White fuming nitric acid is concentrated with 97.5% nitric acid and less than 2% water. It is a colorless to pale-yellow liquid that fumes strongly. It is decomposed by heat and exposure to light and becomes red in color from nitrogen dioxide. Red fuming nitric acid contains more than 85% nitric acid, 6%–15% nitrogen dioxide, and 5% water. The four-digit UN identification number for red fuming nitric acid is 2032. The NFPA 704 designation is health 4, flammability 0, and reactivity 1. The prefix “oxy” appears in the white section of the diamond. Red fuming nitric acid is considered an oxidizer. Both white and red fuming acids are toxic by inhalation, strong corrosives, and dangerous fire risks that may explode upon contact with reducing agents. They are used in the production of nitro compounds, rocket fuels, and as laboratory reagents.m?gliche Exposition
Nitric acid is the second most important industrial acid and its production represents the sixth largest chemical industry in the United States. Nitric acid is used in chemicals, explosives, fertilizers, steel pickling; metal cleaning. The largest use of nitric acid is in the production of fertilizers. Almost 15% of the production goes into the manufacture of explosives, with the remaining 10% distributed among a variety of uses, such as etching, bright-dipping; electroplating, photoengraving, production of rocket fuel; and pesticide manufacture.Carcinogenicity
Nitric acid was not mutagenic in limited studies.4 There is no information regarding the carcinogenic properties of nitric acid, but an association between incidences of laryngeal cancer and exposure to acid mists has been indicated.4Lager
Splash goggles and rubber gloves should be worn when handling this acid, and containers of nitric acid should be stored in a well ventilated location separated from organic substances and other combustible materials.Versand/Shipping
UN2031 Nitric acid other than red fuming, with .70% nitric acid or Nitric acid other than red fuming, with at least 65%, but not >70% nitric acid, Hazard Class: 8; Labels: 8-Corrosive material, 5.1-Oxidizer. UN2032 Nitric acid, red fuming, Hazard Class: 8; Labels: 8-Corrosive material, 5.1-Oxidizer, 6.1-Poisonous material. Inhalation, Hazard Zone B. UN2031 Nitric acid other than red fuming, with >20% and <65% nitric acid or Nitric acid other than red fuming, with not >20% nitric acid, Hazard Class: 8; Labels: 8-Corrosive material.l?uterung methode
The acid is obtained colourless (approx. 92%) by direct distillation of fuming HNO3 under reduced pressure at 40-50o with an air leak at the head of the fractionating column. Store it in a desiccator kept in a refrigerator. Nitrite-free HNO3 can be obtained by vacuum distillation from urea. [Ward et al. Inorg Synth III 13 1950, Kaplan & Schechter Inorg Synth IV 53 1953.]Inkompatibilit?ten
A strong oxidizer and strong acid. Reacts violently with combustible and reducing agents; carbides, hydrogen sulfide, turpentine, charcoal, alcohol, powdered metals; strong bases. Heat causes decomposition producing nitrogen oxides. Attacks some plastics. Corrosive to metals.Waste disposal
Soda ash-slaked lime is added to form the neutral solution of nitrate of sodium and calcium. This solution can be discharged after dilution with water. Also, nitric acid can be recovered and reused in some cases as with acrylic fiber spin solutions. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.Salpetersure Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Platin
Magnesiumnitrat
Kohlenmonoxid
Compressor
cellular membranes
Electronic grade nitric acid
Air blower
Sauerstoff
Distickstofftetroxid
Stickstoffmonoxid
Industrial nitric acid
Dehydrolyzing agent
Concentrated sulfuric acid
Heat exchanger
Magnesiumoxid
Natriumnitrat
Ammoniak, wasserfrei
Downstream Produkte
Bleimetaborat
PYRIDINE-2-SULFONIC ACID
4-Hydroxy-3,5-dinitrobenzaldehyd
2-NITROTHIOPHENE-4-CARBOXALDEHYDE
1,3-Dimethyl-8-nitro-1H-purine-2,6(3H,9H)-dione ,97%
Lanthanum(III) nitrate hexahydrate
3-Amino-2,4,6-triiod-5-[(methylamino)carbonyl]benzoesure
CADMIUM NITRATE TETRAHYDRATE
5-Methylisoxazol-3-carboxylsure
2-Nitromesitylen
8-Hydroxy-5,7-dinitronaphthalin-2-sulfonsure
2-METHYL-5-NITRO-PYRIMIDINE-4,6-DIOL
5-Chloro-2-hydroxy-3-nitropyridine
Dikaliumtetraiodomercurat
3-Nitrobenzonitril
2-Nitrothiophen
4-Acetamido-3-nitrobenzoesure
6-Nitropiperonal
Diquecksilberdichlorid
Phenylquecksilbernitrat
10-Nitroanthrone
Sodium nitrohumate
2,6-Dimethyl-3-nitropyridin
Diquecksilberdinitrat
2-Hydroxy-5-nitronicotinic acid
5-Nitrobarbitursure
3-METHYLPYRAZINE-2-CARBOXYLIC ACID
2-HYDROXY-3,5-DINITROPYRIDINE
Diquecksilberdiiodid
4,5-Diphenyl-1H-imidazol
2-ethyl-5-nitrobenzenamine
3,3'-DINITROBENZOPHENONE
Mesaconitsure
mercurous bromide
Diethylnitromalonat
Cadmiumnitrat
2,4-Dichloro-5-nitrobenzalehyde
hydrofining catalysts (RN series)
Tetranitromethan
Methyl-3-nitrobenzoat
7697-37-2(Salpetersure)Verwandte Suche:
Stickstoffmonoxid
Natriumnitrit
ACETIC ACID
Glycin
Zitronensure
Folsure
Silberbromid
ASCORBICACID
Bleidinitrat
Salpetersure
Strontiumnitrat
Kaliumnitrat
Miconazolnitrat
Silbernitrat
Natriumnitrat
Ammoniumnitrat
phosphoric acid
- acidenitrique
- AZOTIC ACID
- AQUA FORTIS
- CONTRACT LAB PROGRAM AA/ICP NITRIC ACID REAGENT/MATRIX BLANK SOLUTION
- ELUENT (1MMOL/L HNO3)
- Nitric acid, 70%, redistilled, 99.999+% metals basis
- NITRIC ACID 70% SEMICONDUCTOR GRADE PURA
- NITRIC ACID MIN. 65 %, R. G., REAG. REAG. ISO, REAG. PH. EUR., FOR DET. WITH DIT
- NITRIC ACID STANDARD SOLUTION, 1 M (1 N)
- NITRIC ACID 70% SEMICONDUCTOR GRADE MOS
- NITRIC ACID 90% A.C.S. REAGENT
- NITRIC ACID, VOLUMETRIC STANDARD, 1.0N S OLUTION IN WATER
- NITRIC ACID, 70%, A.C.S. REAGENT (POLY-C OATED BOTTLES)
- CLP AA/ICP NITRIC ACID REAGENT/MATRIX BL ANK SOLUTION, 5% IN ASTM TYPE I H2O
- 0,1 MOL NITRIC ACID FIXANAL
- NITRIC ACID 70% SEMICONDUCTOR GRADE VLSI PURANAL
- Nitric acid approx. 65 %
- NITRIC ACID 65%, PACKED IN COATED GLASS BOTTLE
- NITRIC ACID STANDARD SOLUTION 0.1 MOL/L
- NITRIC ACID STANDARD SOLUTION 1.0 MOL/L
- NitricAcid70%A.R.
- NitricAcid70%ElGrade
- NitricAcidGr
- NitricAcidPure69-72%
- NitricAcidHNO3
- NitricAcid50-70%
- NitricAcidAcs
- acidenitrique(french)
- Acido nitrico
- acidonitrico
- Azotowy kwas
- azotowykwas
- azotowykwas(polish)
- Dynamite acid
- engraver’sacid
- Engravers acid
- Kyselina dusicne
- kyselinadusicne
- kyselinadusicne(czech)
- Nitric acid red fuming
- nitricacid(redfuming)
- nitricacid,[>40%]
- nitricacid,40%orless
- nitricacidtechnical
- nitrousfumes
- Nitryl hydroxide
- nitrylhydroxide
- Red fuming nitric acid
- redfumingnitricacid
- Rfna
- Salpeterzuuroplossingen
- NITRIC ACID, DILUTE R
- NITRIC ACID STANDARD
- NITAL
- NITRIC ACID, HUEY
- NITRIC ACID, 2.000N
- NITRIC ACID, 2.00 NORMAL
- NITRIC ACID, 2.850N