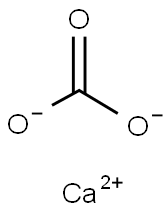
Kalkstein
|
|
Kalkstein Eigenschaften
- Schmelzpunkt:
- 825 °C
- Siedepunkt:
- 2850 °C
- Dichte
- 2.93 g/mL at 25 °C(lit.)
- L?slichkeit
- 5 M HCl: 0.1 M at 20 °C, clear, colorless
- Aggregatzustand
- random crystals
- Farbe
- Gray
- Solubility Product Constant (Ksp)
- pKsp: 8.47
- CAS Datenbank
- 1317-65-3(CAS DataBase Reference)
- EPA chemische Informationen
- Limestone (1317-65-3)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xi | ||
|---|---|---|---|
| R-S?tze: | 37/38-41 | ||
| S-S?tze: | 26-36/37/39 | ||
| OEB | B | ||
| OEL | TWA: 10 mg/m3 (total) | ||
| WGK Germany | - | ||
| RTECS-Nr. | FF9335000 |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Kalkstein Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R37/38:Reizt die Atmungsorgane und die Haut.R41:Gefahr ernster Augensch?den.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Beschreibung
Calcite is one of the most common minerals on the face of the earth, comprising about 4% by weight of the earth’s crust and is formed in many different geological environments. Calcite can form rocks of considerable mass and constitutes a significant part of all three major rock classification types. It forms oolitic, fossiliferous and massive limestones in sedimentary environments and even serves as the internal cement for many sandstones and shales.Calcite is even a major component in the igneous rock called carbonatite and forms the major portion of many hydrothermal veins. Not necessarily a variety of calcite, cave formations are certainly a unique aspect of calcite’s story.Chemische Eigenschaften
Calcium carbonate is a white, odorless powder, or crystalline solid.Verwenden
Source of lime; neutralizing agent, filler, and extender in rubber, plastics, paints; opacifying agent in paper; fortification of bread; putty; tooth pow- ders; antacid; whitewash; Portland cement; sulfur dioxide removal from stack gases; metallurgical flux; analytical chemistry; carbon dioxide gener- ation (laboratory).synthetische
The calcite present is derived mostly from the remains of organisms such as clams, brachiopods, bryozoa, crinoids and corals. These animals live on the bottom of the sea and when they die their shells accumulate into piles of shelly debris. This debris can then form beds of limestone. Some limestones may have been derived from nonbiogenic calcite formation.Definition
Aragonite: An anhydrous mineral form of calcium carbonate, CaCO3, which occurs associated with limestone and in some metamorphic rocks. It is also the main ingredient of pearls. It is not as stable as calcite, into which it may change over time.Calcite:A mineral form of calcium carbonate occurring in limestone, chalk, and marble.
Charlk: A natural form of calcium carbonate (CaCO3) formed originally by marine organisms. (Blackboard chalk is calcium sulfate, CaSO4.).
Allgemeine Beschreibung
Odorless, white to tan powder.Reaktivit?t anzeigen
CALCIUM CARBONATE has generally low chemical reactivity. Is non-combustible. Decomposes at high temperature (825°C) to give gaseous carbon dioxide and calcium oxide (quicklime). Incompatible with acids, alum, ammonium salts, fluorine, magnesium. Reacts with acids and acidic salts to generate gaseous carbon dioxide with effervescence (bubbling). The reaction is rapid and exothermic with concentrated solutions of acids. The efferversence can create extensive foaming. Ignites on contact with fluorine.Hazard
A nuisance particulate dust.Health Hazard
Calcium carbonate is considered to be a nuisance dust.Although no adverse effects have been reported in the literature among workers exposed to calcium carbonate, high concentrations of the dust would be expected to act as a physical irritant to the eyes and skin.1 Fourteen British workers exposed to heavy calcium carbonate concentrations for 12–35 years showed no trace abnormalities due to dust or any clinical sign of pneumoconiosis or chronic bronchitis on X ray.2 Long exposure to high dust concentrations of pure calcium carbonate (quartz content less than 1.1%) did not result in lung fibrosis.
Landwirtschaftliche Anwendung
Limestone or dolomite, used in agriculture for liming the soil, is ground to a fineness to ensure that 50% of the particles pass through a 1.70mm Indian Standards (IS) sieve, and 50% is retained on a 150micron IS sieve.Sicherheitsprofil
A nuisance dust. An eye and skin irritant. Igmtes on contact with F2. Incompatible with acids, ammonium salts, (Mg + H2). Calcium carbonate is a common air contaminant. See also CALCIUM COMPOUNDS.m?gliche Exposition
Calcium carbonate is used as a source of lime; neutralizing agent; manufacturing or rubber, plastics, paint and coatings; sealants, paper, dentifrices, ceramics, putty, polishes and cleaners, insecticides, inks and cosmetics; whitewash; Portland cement; antacids; analytical chemistry, and othersInkompatibilit?ten
Calcium carbonate decomposes in high temperature forming carbon dioxide and corrosive materialsWaste disposal
Landfills. It is the responsibility of chemical waste generators to determine if a discarded chemical is classified as a hazardous waste. See 40 CFR Parts 261.3 for United States Environmental Protection Agency guidelines for the classification determination. In addition, in order to ensure complete and accurate classification, waste generators must consult state and local hazardous waste regulations.Kalkstein Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Regulus silicate cement
Artinite
Sodium tetraborate decahydrate
ammonia synthesis Fe catalysts
Cyanguanidin
Bariumcarbonat
Calciumcarbonat
Natriumdichromat Dihydrat
(+/-)-NICOTINE
Calciumoxid
Diantimontrioxid
Calciumchlorid
Zinkoxid
calcium magnesium phosphate(CMP)
Silicium
Kohlenstoffdioxid
Lithiumcarbonat
Talg (Mg3H2(SiO3)4)
Calciumacetylid
AMMONIA SYNTHESIS CATALYST
Calcium chloride dihydrate
Lithium hydroxide monohydrate
Calciumhydrogenorthophosphat
Calciumnitrit
Cement dust
Calciumsulfat
Natriumhydrogencarbonat
Natriumhydroxid
Glas, Oxid, Chemikalien
Calciumdi(acetat)
1,2,4-Trichlorbenzol
Calciumhypochlorit
Calcium chloride hexahydrate
Aluminiumhydroxid
Calcium phosphate dibasic dihydrate
Calciumdihydroxid
Aluminiumoxid
Natriumcarbonat
SODA ASH LIGHT 99.2% MIN.
Kalkstein Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 130)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 |
info@longyupharma.com | China | 2558 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Dorne Chemical Technology co. LTD | +86-86-13583358881 +8618560316533 |
Ethan@dornechem.com | China | 3097 | 58 |
| Hebei Qige Biological Technology Co. Ltd | +86 +8618733132031 |
CHINA | 1303 | 58 | |
| Henan Alfa Chemical Co., Ltd | |
China | 12005 | 58 | |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 |
sales06@hbduling.cn | China | 8051 | 58 |
1317-65-3(Kalkstein)Verwandte Suche:
- PARIS WHITE
- CALCIUM CARBONATE 325
- WHITING
- lime stones
- CHALK, PRECIPITATED
- ENGLISH WHITE
- KALKSPAR
- ICELAND SPAR
- FORMAXX(R) CALCIUM CARBONATE
- CALCIUM (II) CARBONATE
- CALCIUM CARBONATE, LIGHT
- CALCII CARBONAS
- CALCIUM CARBONATE FROM OYSTER SHELL
- LIME STONE POWDER
- CALCIUM CARBONATE (FOOD GRADE)
- CALCIUM CARBONATE POWDER, 99.50(CACO3)
- CALCIUM CARBONATE LIGHT (U-208)
- CHALKPOWDER
- MARBLEDUST
- CALCIUMCARBONATEMINERAL
- Calciumcarbonat
- agricultural limestone
- Agstone
- bell mine pulverized limestone
- domolite
- flux lime
- franklin
- lithograpic stone
- natural calcium carbonate
- MAGGRAN(R) CCPLUS
- MAGGRAN(R) CC
- MAGNESIA 84460
- MAGNESIA 84472
- MAGNESIA 84470
- MAGNESIA 84479
- MARBLE CHIPS
- portland stone
- sohnhofen stone
- VICRON 15-15 FG LIMESTONE
- CalciumCarbonateCaCO3
- Micromarble
- Calcium carbonate- respirable dust
- 99% purity CALCIUM CARBONATE
- PRECIPITATED CHALK
- GROUND LIMESTONE
- CALCITE
- ARAGONITE
- CHALK
- 1317-65-3
- 472-34-1
- Alphabetic
- Synthetic Reagents
- Inorganic Bases
- CA - CG
- Analytical Standards
- Analytical Chromatography Product Catalog