| Identification | More | [Name]
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl | [CAS]
76189-55-4 | [Synonyms]
(+/-)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE)
(+/-)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHALENE
(+/-)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
(+/-)-BINAP
BINAP
R(+)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE)
R-(+)-1,1'-BINAPHTHYL-2,2'-DIPHENYL PHOSPHINE
R(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHALENE
(R)-(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
(R)-(+)-2,2BIS(DIPHENYLPHOSPHINO)-1,1-BINAPHTHYL
(R)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RAC-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RAC-2,2-BIS(DIPHENYLPHOSPHINO)-1,1-BINAPHTHYL
RAC-BINAP
RACEMIC-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL
RACEMIC-BINAP
(R)-(+)-BINAP
(R)-BINAP
S(-)-(1,1'-BINAPHTHALENE-2,2'-DIYL)BIS(DIPHENYLPHOSPHINE) | [EINECS(EC#)]
616-304-7 | [Molecular Formula]
C44H32P2 | [MDL Number]
MFCD00010805 | [Molecular Weight]
622.67 | [MOL File]
76189-55-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powde | [Melting point ]
283-286 °C(lit.)
| [alpha ]
240 º (c=0.3, toluene) | [Boiling point ]
724.3±55.0 °C(Predicted) | [refractive index ]
235 ° (C=0.3, Toluene) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Benzene (Slightly), Chloroform (Slightly) | [form ]
Powder | [color ]
White to cream-white | [optical activity]
[α]20/D +222°, c = 0.5% in benzene | [Water Solubility ]
insoluble | [Sensitive ]
Air Sensitive | [Merck ]
14,1223 | [BRN ]
4914063 | [InChIKey]
MUALRAIOVNYAIW-UHFFFAOYSA-N | [SMILES]
P(C1C=CC=CC=1)(C1C=CC=CC=1)C1C=CC2=CC=CC=C2C=1C1C2=CC=CC=C2C=CC=1P(C1C=CC=CC=1)C1C=CC=CC=1 | [CAS DataBase Reference]
76189-55-4(CAS DataBase Reference) | [Storage Precautions]
Store under nitrogen;Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [F ]
8-10-23 | [TSCA ]
No | [HS Code ]
29319099 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light yellow crystal powde | [Uses]
Chiral ligand for metal mediated asymmetric catalysis. | [General Description]
(R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene is an axially dissymmetric bis(triaryl)phosphine ligand for asymmetric reactions. |
| Questions And Answer | Back Directory | [Reaction]
- (R)-BINAP or (R)-Tol-BINAP can be combined with dichloro(1,5-cyclooctadiene)ruthenium to form precursors to NOYORI CATALYST SYSTEMS. These systems exhibit very high catalytic activity and enantioselectivity in the hydrogenation of a wide range of substrates. NOYORI CATALYST SYSTEMS have been shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydroxy, siloxy, carbonyl, ester, amide or thioester.
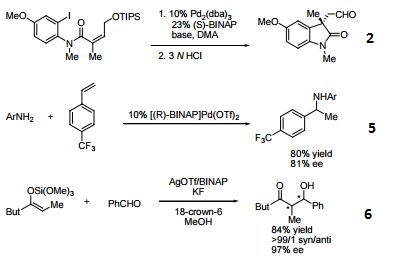
- Useful ligand in asymmetric Heck processes.
- Ligand employed in palladium-catalyzed asymmetric arylation of ketones.
- Ligand employed in rhodium-catalyzed 1,4-additions to enones.
- Ligand employed in palladium-catalyzed hydroamination of styrene derivatives.
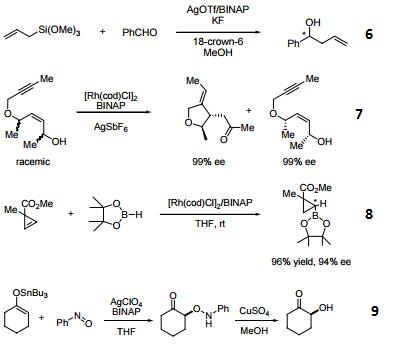
- Ligand employed in silver-catalyzed asymmetric Sakuri-Hosomi allylation and Mukaiyama aldol reaction.
- Ligand employed in rhodium-catalyzed kinetic resolution of enynes.
- Ligand employed in asymmetric rhodium-catalyzed hydroboration of cyclopropenes.
- Ligand employed in silver-catalyzed a-hydroxylation of stannyl enol ethers.
- Ligand employed in palladium-catalyzed synthesis of chiral allenes.
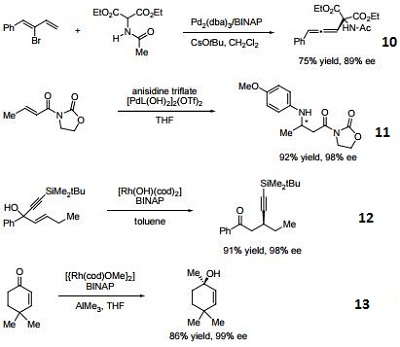
- Ligand for palladium-catalyzed enantioselective hetero Michael addition to form b-amino acid derivatives.
- Ligand employed in rhodium-catalyzed asymmetric rearrangement of alkynyl alkenyl carbinols.
- Ligand employed in rhodium-catalyzed 1,2-addition of aluminium organyl compounds to cyclic enones.
- Ligand employed in iridium-catalyzed transfer hydrogenative allylation of benzylic alcohols.
- Ligand employed in rhodium-catalyzed asymmetric C-Si bond formation by conjugate silyl transfer using a Si-B linkage.
- Ligand employed in the iridium-catalyzed asymmetric cyclopropane-mediated carbonyl allylation of primary alcohols.
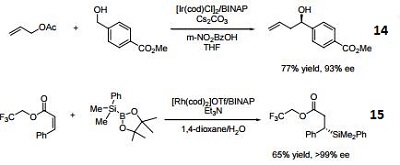
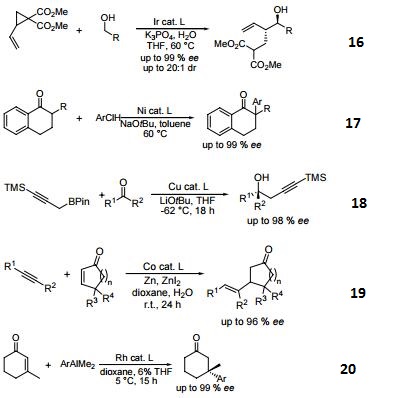
- Ligand employed in the nickel-catalyzed asymmetric α-arylation of tetralones.
- Ligand employed in the copper-catalyzed asymmetric propargylation of ketones.
- Ligand employed in the cobalt-catalyzed asymmetric reductive coupling of alkynes with alkenes.
- Ligand employed in the rhodium-catalyzed asymmetric 1,4-addition of arylalanes on trisubstituted enones.
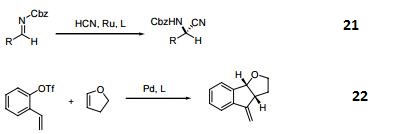
- Ruthenium-catalyzed asymmetric hydrocyanation of imines.
- Palladium-catalyzed asymmetric intermolecular cyclization.
|
| Spectrum Detail | Back Directory | [Spectrum Detail]
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)1HNMR
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)13CNMR
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)31PNMR
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)IR1
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)IR2
(R)-(+)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl(76189-55-4)Raman
|
|
|