| Identification | More | [Name]
1,1'-Bis(diphenylphosphino)ferrocene | [CAS]
12150-46-8 | [Synonyms]
1,1'-BIS(DIPHENYLPHOSPHINO)FERROCENE
1,1'-FERROCENEBIS(DIPHENYLPHOSPHINE)
1,1'-FERROCENEDIYL-BIS(DIPHENYLPHOSPHINE)
DPPF
1,1'-bis(diphenyphosphino)ferrocene
1,1'-bis(diphenyphosphino)ferrocene(dppf)
1,1'-BIS(DIPHENYLPHOSPHINO)FERROCENE, 97 %
1,1'-Bis(diphenylphosphino)ferrocene,99%DPPF
1,1''-BIS(DIPHENYLPHOSPHINO)ERROCENE
1,1''-BIS(DIPHENYLPHOSPHINO)FERROCENE (DPPF)
DPPF/1,1''-BIS(DIPHENYLPHOSPHINO) FERROCENE
1,1''-BIS-(DIPHENYLPHOSPHINO)-FEROCENE
1,1'-Bis(diphenylphosphino) ferrocene, Catalyst Grade
1,1'-Bis(diphenylphosphino)ferrocene, C 73.4%, H 5.2%
1,1μ-Ferrocenebis(diphenylphosphine), 1,1μ-Ferrocenediyl-bis(diphenylphosphine) | [EINECS(EC#)]
430-420-3 | [Molecular Formula]
C34H28FeP2 10* | [MDL Number]
MFCD00001422 | [Molecular Weight]
554.38 | [MOL File]
12150-46-8.mol |
| Chemical Properties | Back Directory | [Appearance]
deep yellow crystalline powder | [Melting point ]
181-182 °C (dec.)(lit.)
| [storage temp. ]
2-8°C
| [solubility ]
Chloroform, Ethyl Acetate | [form ]
crystal | [color ]
yellow to orange | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
Soluble in chloroform, dichloromethane, alcohol and pentane. Insoluble in water. | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [Sensitive ]
Air Sensitive | [Detection Methods]
HPLC | [CAS DataBase Reference]
12150-46-8(CAS DataBase Reference) | [NIST Chemistry Reference]
| [Storage Precautions]
Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi,Xn | [Risk Statements ]
R25:Toxic if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
3467 | [WGK Germany ]
3
| [F ]
10-23 | [TSCA ]
No | [HazardClass ]
IRRITANT | [HS Code ]
29319099 |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Sodium hydroxide-->Ethyl acetate-->Tetrahydrofuran-->Dichloromethane-->n-Butyllithium-->N,N,N',N'-Tetramethylethylenediamine-->Ferrocene-->Dicyclopentadiene-->Ferrous chloride-->Chlorodiphenylphosphine-->Lithium, [1-(diphenylphosphino)-2,4-cyclopentadien-1-yl]--->Diphenylphosphinoferrocene-->1,1'-bis(diphenylphosphinyl)-Ferrocene-->4,4'-Bis(t-butyl)-1,1',2,2'-tetrakis(diphenylphosphino)ferrocene, 98% HiersoPHOS-5-->DIMETHYLPHENYLPHOSPHINE-->Sodium thiocyanate-->Sodium diethyldithiocarbamate-->Phenyllithium | [Preparation Products]
[1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)-->N,N'-Bis- (1-naphthalenyl)-N,N'-bis-phenyl-(1,1'-biphenyl)-4,4'-diamine-->1,1'-Bis(diphenylphosphino)ferrocene-palladium(II)dichloride dichloromethane complex-->[1,1'-Bis(diphenylphosphino)ferrocene]dichloronickel(II) |
| Hazard Information | Back Directory | [Chemical Properties]
1,1'-Bis(diphenylphosphino)ferrocene is deep yellow crystalline powder
| [Uses]
1,1'-Bis(diphenylphosphino)ferrocene used coordination compound in synthesis, readily forms complexes with various metals, i.e. when reacting with the acetonitrile or benzonitrile complexes of PdCl2 it forms (dppf)PdCl2, which i s a popular reagent for palladium-catalyzed coupling reactions.
| [Uses]
suzuki reaction | [General Description]
Novel functionalized furan derivatives were prepared via Pd-phosphine sequential C-C and C-O bond formation. | [reaction suitability]
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carbonylations
reagent type: ligand
reaction type: Ene Reaction
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Tsuji-Trost Reaction | [Purification Methods]
Wash it with distilled H2O and dry it in a vacuum. Dissolve it in ca 5 parts of hot dioxane and cool to give orange crystals m 181-183o. Recrystallisation from *C6H6/heptane (1:2) gives a product with m 183-184o. [Bishop et al. J Organomet Chem 27 241 1971.] |
| Questions And Answer | Back Directory | [Reaction]
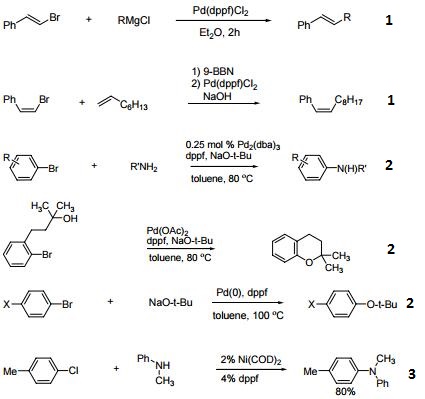
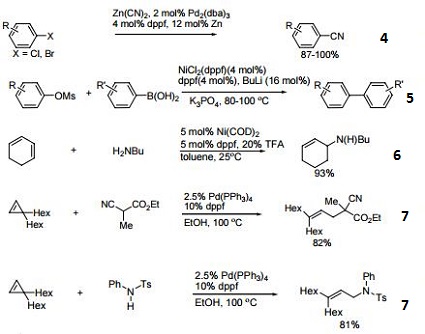
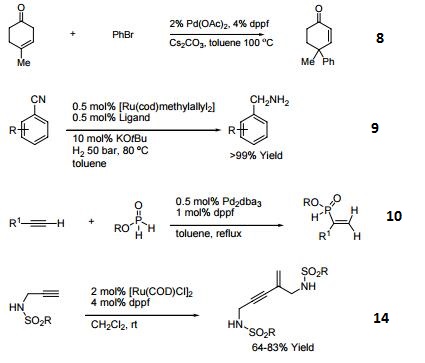
- Ligand for Pd-catalyzed cross-coupling.
- Useful ligand for Pd-catalyzed carbon-nitrogen and carbon-oxygen bond forming procedures.
- Ligand for Ni-catalyzed amination of aryl chlorides.
- Ligand for Pd-catalyzed conversion of aryl halides to aryl nitriles.
- Ligand for Ni-catalyzed Suzuki reactions.
- Ni-catalyzed hydroamination of 1,3-dienes.
- Pd-catalyzed hydrocarbonation and hydroamination of 3,3-dihexylcyclopropene.
- Pd-catalyzed γ-arylation of β,γ-unsaturated ketones.
- Ligand for Ru-catalyzed reduction of nitriles to primary amines.
- Ligand for Rh-catalyzed alkyne head-to-tail dimerization.
- Ligand for Rh-catalyzed cross-coupling
- Ligand for Rh-catalyzed olefin isomerization
- Ligand for Ni or Rh-catalyzed borylation
- Ligand for regioselective Pd-catalyzed hydrophosphinylation of terminal alkynes to form branched alkenes.
|
|
|