| Identification | More | [Name]
(S)-(-)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL | [CAS]
99646-28-3 | [Synonyms]
(R)-(+)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL
(R)-(+)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)1,1-BINAPHTHYL
(R)-TOL-BINAP
(S)-(-)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL
(S)-TOL-BINAP
(R)-(+)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl, (R)-p-Tol-BINAP
(R)-(+)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl, 95+%
(R)-(+)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl,98%(R)-Tol-BINAP
(R)-(+)-2,2''-BIS(DI-P-TOLYLPHOSPHINO)-1,1''-BINAPHTHYL (R)-TOL-BINAP
PHOSPHINE, 1,1''-(1R)-[1,1''-BINAPHTHALENE]-2,2''-DIYLBIS[BIS(4-METHYLPHENYL)-
(R)-T-BINAP
(R)-Tol-BINAP, (R)-(+)-2,2μ-Bis(di-p-tolylphosphino)-1,1μ-binaphthyl | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C48H40P2 | [MDL Number]
MFCD00269856 | [Molecular Weight]
678.78 | [MOL File]
99646-28-3.mol |
| Chemical Properties | Back Directory | [Appearance]
White to cream powder | [Melting point ]
255-257 °C
| [alpha ]
+156° (c 0.5, C6H6) | [Boiling point ]
754.4±60.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
Powder | [color ]
White to cream | [optical activity]
[α]20/D +162°, c = 0.5 in benzene | [Water Solubility ]
Insoluble in water. | [λmax]
223nm(EtOH)(lit.) | [InChIKey]
IOPQYDKQISFMJI-UHFFFAOYSA-N | [SMILES]
P(C1C=CC(C)=CC=1)(C1C=CC(C)=CC=1)C1C=CC2=CC=CC=C2C=1C1C2=CC=CC=C2C=CC=1P(C1C=CC(C)=CC=1)C1C=CC(C)=CC=1 | [CAS DataBase Reference]
99646-28-3(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive;Store under inert gas;Air sensitive |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3 | [TSCA ]
No | [HS Code ]
29310099 |
| Hazard Information | Back Directory | [Chemical Properties]
White to cream powder | [Uses]
(R)-(+)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl is used as chiral catalyst ligand. (R)-T-BINAP complexes derived from rhodium precursors are used for the asymmetric hydroformylation of vinyl acetate. It is a catalysts used for reductive amination of ketones, Pt dications for cation trapping, Rh(I)-catalyst for hydrogenation of acetamidoacrylic acid derivatives. | [General Description]
BINAP is based on a bis naphthalene backbone with different phosphine derivatives. 2,2′-bis(di-p-tolylphosphino)-1,1′-binaphthyl (p-Tol-BINAP)·AgF complex catalyzed asymmetric Mukaiyama-type aldol reaction is reported. | [reaction suitability]
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings |
| Questions And Answer | Back Directory | [Reaction]
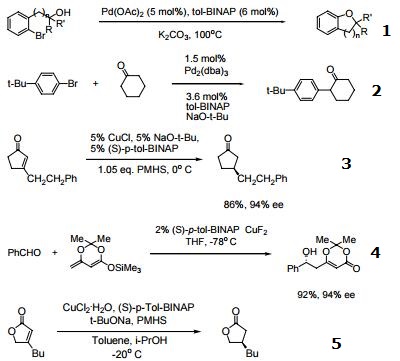
- Useful ligand for palladium-catalyzed carbon-oxygen bond formation.
- Ligand for palladium-catalyzed α-arylation of ketones.
- Ligand for Cu-catalyzed asymmetric conjugate reduction.
- Ligand for Cu-catalyzed asymmetric dienolate addition to aldehydes.
- Enantioselective conjugate reduction of lactones and lactams.
- Ligand used in the enantioselective cycloaddition of allenylsilanes with α-Imino esters.
- Catalytic Aldol reaction to ketones.
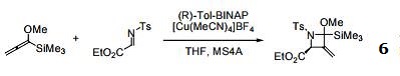
- Ligand with rhodium catalyses [2+2+2] cycloaddition reaction of alkenes and alkynes.
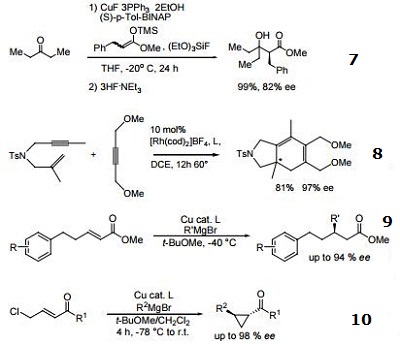
- Ligand used in the copper-catalyzed asymmetric conjugate addition of alkyl Grignard reagents on α,β-unsaturated esters.
- Ligand used in the copper-catalyzed asymmetric synthesis of cyclopropanes via tandem conjugate addition and intramolecular enolate trapping.
|
|
|