| Identification | More | [Name]
(S)-(-)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL | [CAS]
100165-88-6 | [Synonyms]
(R)-(+)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL
(R)-(+)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)1,1-BINAPHTHYL
(R)-TOL-BINAP
(S)-(-)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-BINAPHTHYL
(S)-TOL-BINAP
BisdiptolylphosphinobinaphthylSTolBI
(S)-(-)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl, (S)-p-Tol-BINAP
(S)-2,2'-BIS(DI-P-TOLYLPHOSPHINO)-1,1'-&
(S)-(-)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl, 98+%
(S)-(-)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl,98%(S)-Tol-BINAP
(S)-(-)-2,2''-BIS(DI-P-TOLYLPHOSPHINO)-1,1''-BINAPHTHYL (S)-TOL-BINAP
(S)-(-)-2,2''-Bis-[bis-(p-tolylphosphino)]-1,1''-binaphthyl
PHOSPHINE, 1,1''-(1S)-[1,1''-BINAPHTHALENE]-2,2''-DIYLBIS[1,1-BIS(4-METHYLPHENYL)-
(S)-T-BINAP
(S)-Tol-BINAP, (S)-(-)-2,2μ-p-tolyl-phosphino)-1,1μ-binaphthyl | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C48H40P2 | [MDL Number]
MFCD00269856 | [Molecular Weight]
678.78 | [MOL File]
100165-88-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
252-256 °C
| [alpha ]
-160° (c 0.5, C6H6) | [Boiling point ]
754.4±60.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [form ]
Powder | [color ]
White | [optical activity]
[α]20/D -156°, c = 0.5 in benzene | [Water Solubility ]
Insoluble in water. | [InChIKey]
IOPQYDKQISFMJI-UHFFFAOYSA-N | [CAS DataBase Reference]
100165-88-6(CAS DataBase Reference) | [Storage Precautions]
Air sensitive;Moisture sensitive;Store under inert gas |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3 | [TSCA ]
No | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
(S)-(-)-2,2'-Bis(di-p-tolylphosphino)-1,1'-binaphthyl is chiral ligand widely used in asymmetric synthesis. It also used as excellent catalysts for asymmetric hydrogenation of alkenes and some cyclic anhydrides. BINAP is used in organic synthesis for enantioselective transformations catalyzed by its complexes of ruthenium, rhodium, and palladium |
| Questions And Answer | Back Directory | [Reaction]
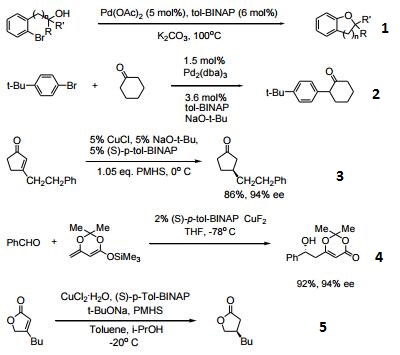
- Useful ligand for palladium-catalyzed carbon-oxygen bond formation.
- Ligand for palladium-catalyzed α-arylation of ketones.
- Ligand for Cu-catalyzed asymmetric conjugate reduction.
- Ligand for Cu-catalyzed asymmetric dienolate addition to aldehydes.
- Enantioselective conjugate reduction of lactones and lactams.
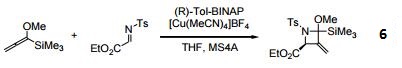
- Ligand used in the enantioselective cycloaddition of allenylsilanes with α-Imino esters.
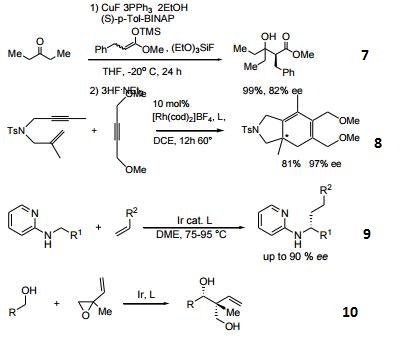
- Catalytic Aldol reaction to ketones.
- Ligand with rhodium catalyses [2+2+2] cycloaddition reaction of alkenes and alkynes.
- Ligand used in the iridium-catalyzed enantioselective C-H bond activation of 2-(alkylamino)-pyridine with alkenes.
- Iridium-catalyzed regio-, diastereo-, and enantioselective tert-(hydroxyl)-prenylation of alcohols.
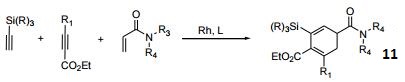
- Rhodium-catalyzed cross cyclotrimerization.
|
|
|