| Identification | More | [Name]
Amiodarone | [CAS]
1951-25-3 | [Synonyms]
2-BUTYL-3-BENZOFURANYL 4-[2-(DIETHYLAMINO)-ETHOXY]-3,5-DIIODOPHENYL KETONE
AMIODARONE
(2-butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diidophenyl)methanone
2-butyl-3-(3,5-diiodo-4-(2-diethylaminoethoxy)benzoyl)benzofuran
2-butyl-3-(3,5-diiodo-4-(beta-diethylaminoethoxy)benzoyl)benzofuran
2-butyl-3-(3,5-diiodo-4-(beta-diethylaminoethoxy)-benzoyl)benzofuran
2-butyl-3-(4’-beta-n-diethylaminoethoxy-3’,5’-diiodobenzoyl)benzofuran
2-butyl-3-benzofuranylp-((2-diethylamino)ethoxy)-m,m-diiodophenylketone
2-n-butyl-3’,5’-diiodo-4’-n-diethylaminoethoxy-3-benzoylbenzofuran
amiodarona
ketone,2-butyl-3-benzofuranyl4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl
methanone,(2-butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophen
Amiodarone Base
Amiodarons
Methanone, (2-butyl-3-benzofuranyl)4-2-(diethylamino)ethoxy-3,5-diiodophenyl-
Atlansil
Tranquerone
(2-Butylbenzofuran-3-yl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone
Sedacoron
Sedacorone | [EINECS(EC#)]
217-772-1 | [Molecular Formula]
C25H29I2NO3 | [MDL Number]
MFCD00242801 | [Molecular Weight]
645.31 | [MOL File]
1951-25-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
54 - 55°C | [Boiling point ]
635.1±55.0 °C(Predicted) | [density ]
1.5730 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
6.56(at 25℃) | [color ]
Colourless to Pale Yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
716.4mg/L(25 ºC) | [InChI]
InChI=1S/C25H29I2NO3/c1-4-7-11-22-23(18-10-8-9-12-21(18)31-22)24(29)17-15-19(26)25(20(27)16-17)30-14-13-28(5-2)6-3/h8-10,12,15-16H,4-7,11,13-14H2,1-3H3 | [InChIKey]
IYIKLHRQXLHMJQ-UHFFFAOYSA-N | [SMILES]
C(C1C2=CC=CC=C2OC=1CCCC)(C1=CC(I)=C(OCCN(CC)CC)C(I)=C1)=O | [CAS DataBase Reference]
1951-25-3(CAS DataBase Reference) | [EPA Substance Registry System]
Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]- (1951-25-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
OB1361000
| [Safety Profile]
Poison by intravenous and intraperitoneal routes. Human systemic effects by ingestion: photosensitivity of the skin. A flammable liquid. When heated to decomposition it emits very toxic fumes of Iand NO,. A coronary vasoddator |
| Hazard Information | Back Directory | [Description]
From the chemical point of view, amiodarone is completely different from other antiarrhythmics.
It has two iodide atoms and a diethylaminoethanol group as substituents in the
benzoyl part, and overall it is very similar to the structure of thyroxin-like molecules. | [Originator]
Cordarone,Labaz,France,1971 | [Uses]
antibacterial | [Definition]
ChEBI: A member of the class of 1-benzofurans that is 1-benzofuran substituted by a butyl group at position 2 and a 4-[2-(diethylamino)ethoxy]-3,5-diiodobenzoyl group at position 3. It is a cardiovascular drug used for the treatment of cardiac dysrhythmias. | [Indications]
Clinical use of amiodarone is limited because of its high toxicity, which consists of cardiac
block, bradycardia, cardiac insufficiency, damaged thyroid gland function, neuropathology,
and increased sensitivity to light, all of which significantly limit use of
amiodarona, and it is only used in therapy for extremely serious tachyarrhythmias such as
reoccurring ventricular fibrillation and hemodynamic unstable ventricular tachycardia, and
only under supervision of a physician in a clinical situation. | [Manufacturing Process]
135 grams of 2-n-butyl-3-(3,5-diiodo-4-hydroxybenzoyl)benzofuran dissolved
in 600 cc of ethyl carbonate were treated with 5.7 grams of sodium in the
form of sodium methoxide in methanol. Then, β-diethylaminoethyl chloride
which had been obtained from 51.6 grams of the hydrochloride in ethyl carbonate was introduced into a suspension of the sodium salt. The mixture
was heated to a temperature of approximately 90°C which was maintained for
approximately 2 hours. The mixture was cooled and allowed to stand
overnight during which time the sodium chloride settled down.
The toluene solution containing diethylaminoethyl ether was extracted with
increasingly diluted aqueous hydrochloric acid solutions while stirring.
Extraction was continued until the alkalized solution produced no further
precipitate. The combined aqueous solutions were washed with ether and then
made strongly alkaline with aqueous sodium hydroxide. Extraction with ether
was carried out three times. The organic layers were washed with water and
then dried over anhydrous potassium carbonate. In order to produce the
hydrochloride, the carbonate was filtered off and then the hydrochloride was
precipitated from the ether solution with an ethereal hydrochloric acid
solution. After the solution had been allowed to stand for a few hours,
decantation was carried out and the syrupy hydrochloride residue was taken
up in 500 cc of boiling acetone. The salt crystallized out by cooling. The
substance was allowed to stand overnight at 0°C, and centrifuged, washed
with ethyl acetate and then with ether and dried. 130 grams of 2-n-butyl-3-
(3,5-diiodo-4-β-N-diethylaminoethoxybenzoyl)benzofuran hydrochloride in the
form of a crystalline powder which melts at 156°C were obtained. | [Brand name]
Cordarone (Wyeth-Ayerst). | [Therapeutic Function]
Coronary vasodilator | [Biological Functions]
Amiodarone (Cordarone) is an iodine-containing benzofuran
derivative identified as a class III agent because it
predominantly prolongs action potentials. Amiodarone
also blocks sodium and calcium channels and is a noncompetitive
β-receptor blocker.Amiodarone is effective
for the treatment of most arrhythmias. Toxicity associated
with amiodarone has led the U. S. Food and Drug
Administration (FDA) to recommend that it be reserved
for use in patients with life-threatening arrhythmias. | [General Description]
Amiodarone, 2-butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl ketone (Cordarone),was introduced as an antianginal agent. It has very pronouncedclass III action and is especially effective in maintainingsinus rhythm in patients who have been treated bydirect current shock for atrial fibrillation. Like class IIIantiarrhythmic drugs, amiodarone lengthens the effective refractoryperiod by prolonging the action potential duration inall myocardial tissues. Amiodarone is eliminated very slowlyfrom the body, with a half-life of about 25 to 30 days after oraldoses. Although the drug has a broad spectrum of antiarrhythmicactivity, its main limitation is a slow onset of action.Drug action may not be initiated for several days, and thepeak effect may not be obtained for several weeks. | [Mechanism of action]
Amiodarone’s antiarrhythmic action is connected to its ability to block K�, Na�, and
Ca2� channels while noncompetitively blocking α- and β-adrenergic receptors of the heart,
thus prolonging the action potential and effective refractive period of atrial cells, atrioventricular
junctions, and ventricles of the heart, which is accompanied by decreased automatism
of sinus node and slowing of atrioventricular conductivity. | [Clinical Use]
Amiodarone has adverse effects involving many differentorgan systems. It also inhibits metabolism of drugscleared by oxidative microsomal enzymes. It contains iodinein its molecular structure and, as a result, has an effecton thyroid hormones. Hypothyroidism occurs in up to 11%of patients receiving amiodarone. The principal effect isthe inhibition of peripheral conversion of T4 to T3. Serumreverse T3 (rT3) is increased as a function of the dose as wellas the length of amiodarone therapy. As a result, rT3 levelshave been used as a guide for judging adequacy of amiodaronetherapy and predicting toxicity. | [Side effects]
Amiodarone’s most significant adverse effects include
hepatitis, exacerbation of arrhythmias, worsening of congestive
heart failure, thyroid dysfunction, and pulmonary
fibrosis. Pulmonary fibrosis is frequently fatal and may
not be reversed with discontinuation of the drug.
Interestingly, despite significant prolongation of the QT
interval, the risk of torsades de pointes is relatively low.
Patients with underlying sinus node dysfunction
tend to have significant worsening of nodal function,
frequently requiring pacemaker implantation. Corneal
microdeposits develop in most adults receiving amiodarone.
As many as 10% of patients complain of halos
or blurred vision. The corneal microdeposits are reversible
with stoppage of the drug.
Photosensitization occurs in 10% of patients. With
continued treatment, the skin assumes a blue-gray coloration.
The risk is increased in patients of fair complexion.
The discoloration of the skin regresses slowly, if
at all, after discontinuation of amiodarone.
Amiodarone inhibits the peripheral and possibly intrapituitary
conversion of thyroxine (T4) to triiodothyronine
(T3) by inhibiting 5 -deiodination. The serum
concentration of T4 is increased by a decrease in its
clearance, and thyroid synthesis is increased by a reduced
suppression of the pituitary thyrotropin T3. The
concentration of T3 in the serum decreases, and reverse
T3 appears in increased amounts.Despite these changes,
most patients appear to be maintained in an euthyroid
state. Manifestations of both hypothyroidism and hyperthyroidism
have been reported.
Tremors of the hands and sleep disturbances in the
form of vivid dreams, nightmares, and insomnia have
been reported in association with the use of amiodarone.
Ataxia, staggering, and impaired walking have
been noted. Peripheral sensory and motor neuropathy
or severe proximal muscle weakness develops infrequently.
Both neuropathic and myopathic changes are
observed on biopsy. Neurological symptoms resolve or
improve within several weeks of dosage reduction. | [Synthesis]
Amiodarone, 2-butyl-3-benzofuranyl-4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl
ketone (18.1.21), is synthesized in the following manner. Benzofuran is acylated by
butyric acid anhydride in the presence of phosphorous acid, forming 2-butyroylbenzfuran
(18.1.16). Reduction of the carbonyl group in a Wolff¨CKizhner reaction using hydrazine
hydrate gives 2-butylbenzofurane (18.1.17). This is acylated with 4-methoxybenzoic acid
chloride, giving 2-butyl-3-(4-methoxybenzoyl)benzofuran (18.1.18), which undergoes
demethylation by pyridine hydrochloride, forming 2-butyl-3-(4-hydroxy-benzoyl)-benzofuran
(18.1.19). The resulting product is iodized in the presence of potassium iodide, forming
2-butyl-3-benzofuranyl-4-(2-hydroxy-3,5-diiodophenyl) ketone (18.1.20), which is reacted
further with 2-diethylaminoethylchoride, giving desired amiodarone (18.1.21) .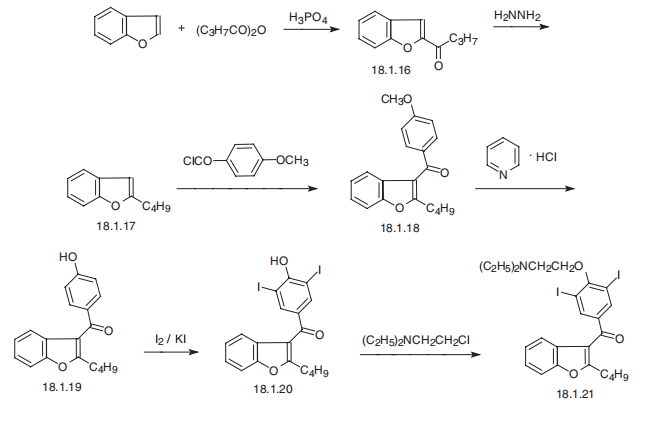
| [Drug interactions]
Amiodarone increases the hypoprothrombinemic response
to warfarin (an oral anticoagulant) by reducing
its metabolism. Patients receiving digoxin may undergo
an increase in serum digoxin concentrations when
amiodarone is added to the treatment regimen.
Amiodarone interferes with hepatic and renal elimination
of flecainide, phenytoin, and quinidine. | [Precautions]
Amiodarone is contraindicated in patients with sick sinus
syndrome and may cause severe bradycardia and secondand
third-degree atrioventricular block. Amiodarone
crosses the placenta and will affect the fetus, as evidenced
by bradycardia and thyroid abnormalities. The
drug is secreted in breast milk. |
|
|