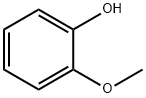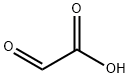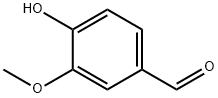
Vanillin synthesis
- Product Name:Vanillin
- CAS Number:121-33-5
- Molecular formula:C8H8O3
- Molecular Weight:152.15
1) Preparation from waste sulfite liquors: The starting material for vanillin production is the lignin present in sulfite wastes from the cellulose industry. The concentrated mother liquors are treated with alkali at elevated temperature and pressure in the presence of oxidants. The vanillin formed is separated from the by-products, particularly acetovanillone (4-hydroxy-3- methoxyacetophenone), by extraction, distillation, and crystallization. A large number of patents describe various procedures for the (mainly) continuous hydrolysis and oxidation processes, as well as for the purification steps required to obtain high-grade vanillin . Lignin is degraded either with sodium hydroxide or with calcium hydroxide solution and simultaneously oxidized in air in the presence of catalysts. When the reaction is completed, the solid wastes are removed. Vanillin is extracted from the acidified solutionwith a solvent (e.g., butanol or benzene) and reextractedwith sodium hydrogen sulfite solution. Reacidification with sulfuric acid followed by vacuum distillation yields technical-grade vanillin, which must be recrystallized several times to obtain food-grade vanillin.Water, to which some ethanol may be added, is used as the solvent in the last crystallization step.
2) Preparation from guaiacol: Severalmethods can be used to introduce an aldehyde group into an aromatic ring. Condensation of guaiacol with glyoxylic acid followed by oxidation of the resulting mandelic acid to the corresponding phenylglyoxylic acid and, finally, decarboxylation continues to be a competitive industrial process for vanillin synthesis.
a. Vanillin from guaiacol and glyoxylic acid: Currently, guaiacol is synthesized from catechol, which is mainly prepared by acid-catalyzed hydroxylation of phenol with hydrogen peroxide. In China, a guaiacol prepared from o-nitrochlorobenzene via o-anisidine is also used. Glyoxylic acid is obtained as a by-product in the synthesis of glyoxal from acetaldehyde and can also be produced by oxidation of glyoxal with nitric acid. Condensation of guaiacol with glyoxylic acid proceeds smoothly at room temperature and in weakly alkaline media. A slight excess of guaiacol is maintained to avoid formation of disubstituted products; excess guaiacol is recovered. The alkaline solution containing 4-hydroxy- 3-methoxymandelic acid is then oxidized in air in the presence of a catalyst until the calculated amount of oxygen is consumed [358]. Crude vanillin is obtained by acidification and simultaneous decarboxylation of the (4-hydroxy-3-methoxyphenyl)glyoxylic acid solution.
This process has the advantage that, under the reaction conditions, the glyoxyl radical enters the aromatic guaiacol ring almost exclusively para to the phenolic hydroxy group. Tedious separation procedures are thus avoided. b. Vanillin from guaiacol and formaldehyde: An older process that is still in use consists of the reaction of guaiacolwith formaldehyde or formaldehyde precursors such as urotropine, N,N-dimethyl-aniline, and sodium nitrite .
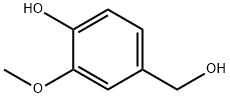
498-00-0
344 suppliers
$21.00/25g

121-33-5
1150 suppliers
$9.00/1g
Yield:121-33-5 100%
Reaction Conditions:
with dihydrogen peroxide in acetonitrile at 40; for 8 h;Catalytic behavior;Reagent/catalyst;
Steps:
Also, for the vanillin alcohol oxidation reaction, 8 mL of acetonitrile was poured into a carouseltube and the temperature was increased to 40 C to provide suitable conditions for complete dissolutionof vanillyl alcohol. Then 0.77 g (5 mmol) of vanillyl alcohol was added to the solvent and after completedissolution of vanillyl alcohol in acetonitrile, 1.2 mL of hydrogen peroxide with 0.1 g catalyst wereadded to the reaction mixture. The carousel was adjusted to 90 C with a magnetic stirrer speed of800 rpm. To evaluate the progress of the reactions, the reaction mixture was sampled at dierent times.Sampling was done by a syringe with a filter. To study the reusability of the samples, catalysts wereseparated after reaction by a paper filter and washed by acetonitrile. Then, they were oven dried at110 C for 24 h and re-used in the vanillin production reaction. To obtain conversion and selectivity ofthe reaction, products were analyzed by the same gas chromatograph and also, results were confirmedby GC-MS.
References:
Rahmanivahid, Behgam;de Dios, Maria Pinilla;Haghighi, Mohammad;Luque, Rafael [Molecules,2019,vol. 24,# 14,art. no. 2597]
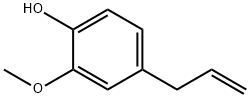
97-53-0
749 suppliers
$5.00/25g

121-33-5
1150 suppliers
$9.00/1g
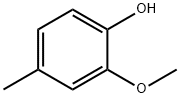
93-51-6
396 suppliers
$15.00/5g

121-33-5
1150 suppliers
$9.00/1g
![Benzaldehyde, 4-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-methoxy-](/CAS/20180601/GIF/69404-94-0.gif)
69404-94-0
9 suppliers
inquiry

121-33-5
1150 suppliers
$9.00/1g