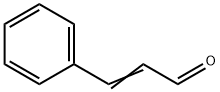
Cinnamaldehyde synthesis
- Product Name:Cinnamaldehyde
- CAS Number:104-55-2
- Molecular formula:C9H8O
- Molecular Weight:132.16
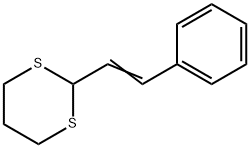
26958-41-8
0 suppliers
inquiry

104-55-2
675 suppliers
$6.00/25g
Yield:104-55-2 94%
Reaction Conditions:
with dimethyl sulfoxide at 20; for 1 h;Reagent/catalyst;Solvent;
Steps:
General procedure for the deprotection of dithiane and dithiolane using PNBA or PNBSas a catalyst in DMSO
General procedure: PNBA (0.035 g, 0.2mmol) or PNBS (0.015 g, 0.1mmol) was added to a solution of thioacetal or thioketal (1mmol) and anhydrous DMSO (0.23 g, 3mmol). The mixture was stirred at room temperature for the appropriate reaction time (Tables 3 and 4). After completion of the reaction (as monitored by TLC), CH2Cl2 was added into the reaction mixture, and it was quenched with a solution of NaOH (5% w/v, 10mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2×25 mL). The combined organic extracts were then washed with brine (1×10 mL), next with water (2×15 mL),and dried over Na2SO4/MgSO4. The almost pure product was prepared after evaporationof the solvent (Tables 3 and 4). If needed, further purification was achieved by column chromatography using EtOAc-n-hexane (1:10) as eluent. The catalyst PNBA or PNBS could berecovered easily by filtration after completion of the reaction, followed by washing with CH2Cl2, and drying at 30°C. The activity of the reused resin was the same as the fresh polymer (Table 5). The identities of products were determined by comparison of thei rphysical and spectral data with those of the authentic samples. All the products are known compounds.
References:
Ebrahimzadeh, Farzaneh [Journal of Sulfur Chemistry,2016,vol. 37,# 1,p. 89 - 104]

64-17-5
756 suppliers
$10.00/50g
100-52-7
970 suppliers
$20.37/250g

104-55-2
675 suppliers
$6.00/25g
621-82-9
360 suppliers
$29.12/100G

104-55-2
675 suppliers
$6.00/25g
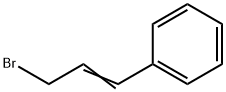
4392-24-9
187 suppliers
$18.00/5g

104-55-2
675 suppliers
$6.00/25g
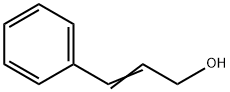
104-54-1
648 suppliers
$13.00/25g

104-55-2
675 suppliers
$6.00/25g