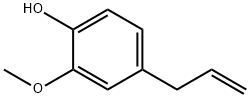
Eugenol synthesis
- Product Name:Eugenol
- CAS Number:97-53-0
- Molecular formula:C10H12O2
- Molecular Weight:164.2
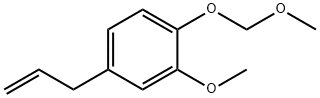
143654-03-9
0 suppliers
inquiry

97-53-0
747 suppliers
$5.00/25g
Yield:97-53-0 96%
Reaction Conditions:
with toluene-4-sulfonic acid in neat (no solvent, solid phase) at 20; for 0.583333 h;Green chemistry;
Steps:
MOM Deprotection by pTSA
General procedure: MOM ether (5 mmol) and pTSA.H2O (7.7 mmol) weretriturated well in a mortar for 5 min (in the case of entry 10trituration time was about 15 min), reaction mixture was leftat room temperature for another 30 min. After completion ofthe reaction (monitored by TLC), cold water (4oC) wasadded. The products were separated by centrifugation. Theyields of the products ranged from 85-98%. The purities andthe identities of the products were established by direct comparisonwith known compounds (TLC, Mp and IR). See supplementaryinformation for further details.
References:
Pandurangan, Nanjan [Letters in Organic Chemistry,2017,vol. 14,# 4,p. 231 - 235]
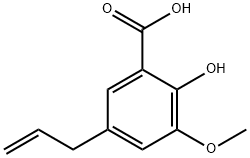
2216-99-1
5 suppliers
inquiry

97-53-0
747 suppliers
$5.00/25g
![Benzene, 1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methoxy-4-(2-propen-1-yl)-](/CAS/20210305/GIF/214330-24-2.gif)
214330-24-2
0 suppliers
inquiry

97-53-0
747 suppliers
$5.00/25g
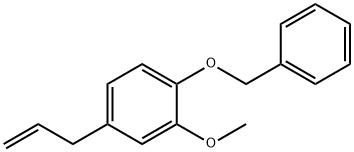
57371-42-3
18 suppliers
inquiry

97-53-0
747 suppliers
$5.00/25g
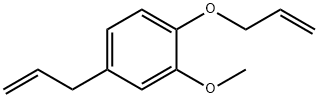
4125-45-5
4 suppliers
inquiry

97-53-0
747 suppliers
$5.00/25g