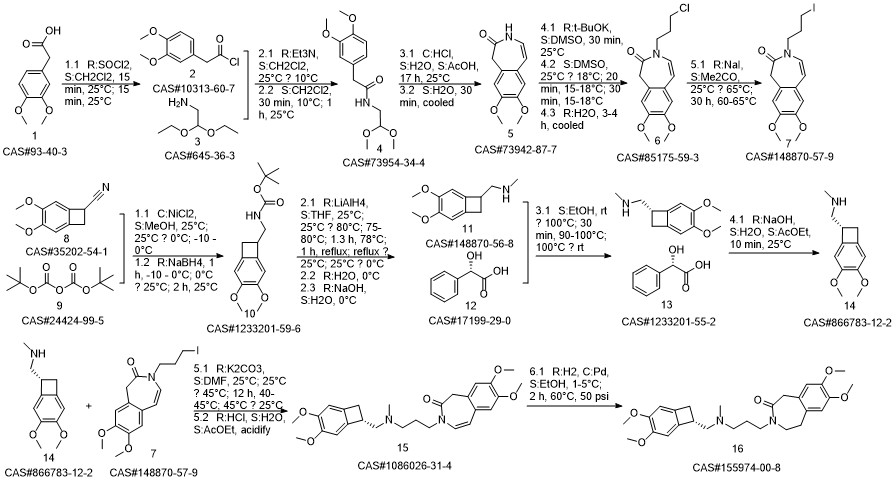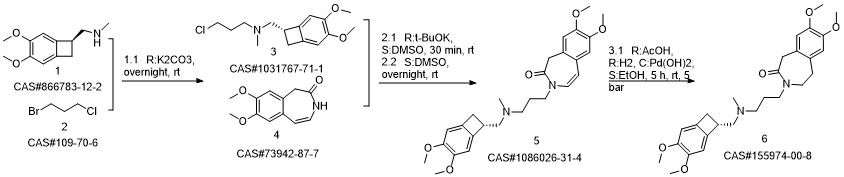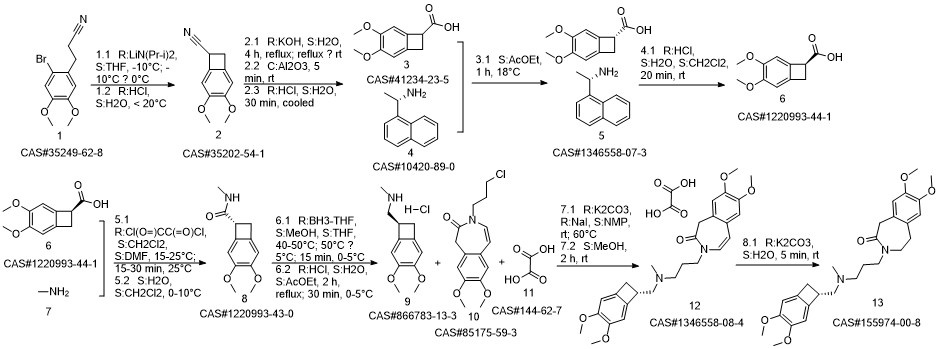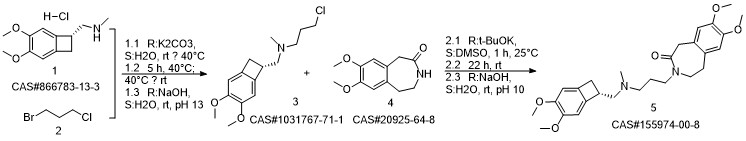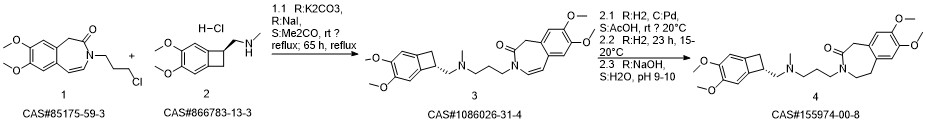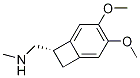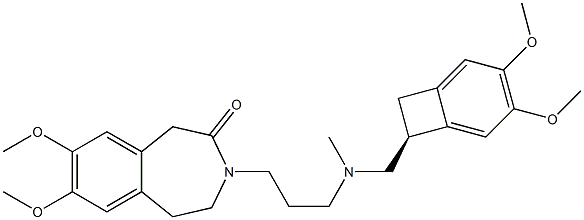
Ivabradine synthesis
- Product Name:Ivabradine
- CAS Number:155974-00-8
- Molecular formula:C27H36N2O5
- Molecular Weight:468.59
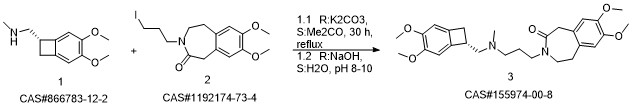
Reference: Pang, Yalong; Liu, Peihua. Process for preparation of Ivabradine and pharmaceutically acceptable salts. CN 101544605. (Assignee Beijing Shenlanhai Bio-Pharmaceutical Science and Technology Co., Ltd., Peop. Rep. China)
![3-[3-[[[(7S)-3,4-DiMethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]Methyl]MethylaMino]propyl]-1,3-dihydro-7,8-diMethoxy-H-3-benzazepin-2-one](/CAS/GIF/1086026-31-4.gif)
1086026-31-4
76 suppliers
$200.00/5mg

155974-00-8
103 suppliers
inquiry
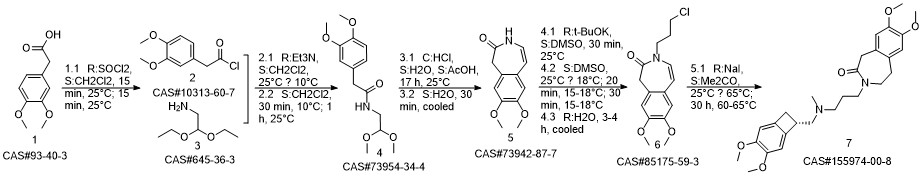
Yield:155974-00-8 96%
Reaction Conditions:
Stage #1: 3-{3-[{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}-(methyl)amino]propyl}-7,8-dimethoxy-1,3-dihydro-2H-3-benzazepin-2-onewith formic acid;palladium 10% on activated carbon;triethylamine in acetonitrile at 50; for 16 h;
Stage #2: with hydrogenchloride in water;acetonitrile at 0 - 5; pH=3 - 3.5;Reagent/catalyst;
Steps:
2 3-3 {- [{[(7S) -3,4-dimethoxybenzocyclobutene-7-] methyl} (methyl) amino-propyl] -7,8- dimethoxy-1,3,4,5-tetrahydro-2-hydrogen-3-benzazepine -2-one hydrochloride} (formic acid and triethylamine)
Under stirring conditions, 46.6 g (0.1 mol) of the oil obtained according to the method of Example 1, 200 ml of acetonitrile, 10 g 10% Pd / C (measured in dry form), 30 g (0.65 mol) of formic acid and 27 g (0.27 mol) of triethylamine were mixed and incubated at 50 ° C for 16 h. The Pd / C was removed by filtration, the volatiles were distilled off under reduced pressure, and then 200 mL of acetonitrile was added, The pH was adjusted to 3.0 to 3.5 by the addition of 36% hydrochloric acid. The mixture was stirred at 0 to 5 ° C for 1 h and then the product was filtered, Dried to obtain 45 g of dry product, HPLC purity of 99.83%, single impurity content of not more than 0.06% The yield was 96%.
References:
CN104788377,2017,B Location in patent:Paragraph 0011; 0026-0027
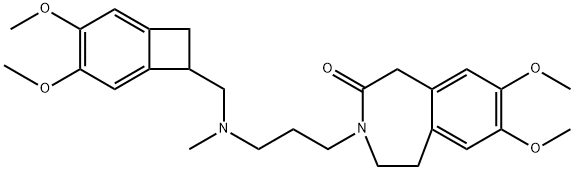
148870-59-1
8 suppliers
inquiry

155974-00-8
103 suppliers
inquiry
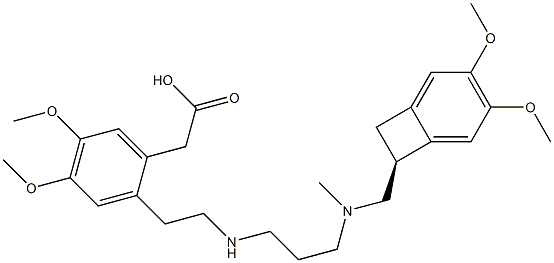
1462470-54-7
34 suppliers
inquiry

155974-00-8
103 suppliers
inquiry
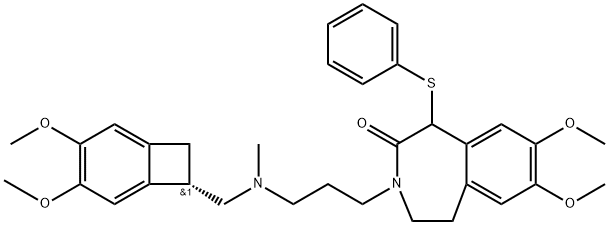
1325209-27-5
1 suppliers
inquiry

155974-00-8
103 suppliers
inquiry