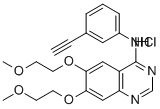
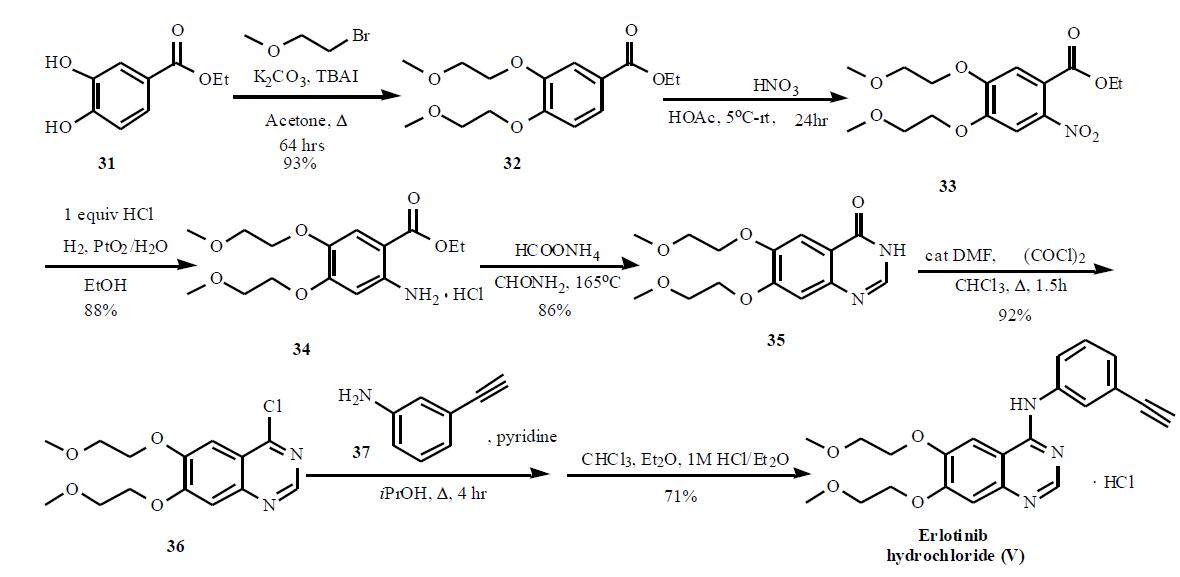
Erlotinib hydrochloride synthesis
- Product Name:Erlotinib hydrochloride
- CAS Number:183319-69-9
- Molecular formula:C22H24ClN3O4
- Molecular Weight:429.9
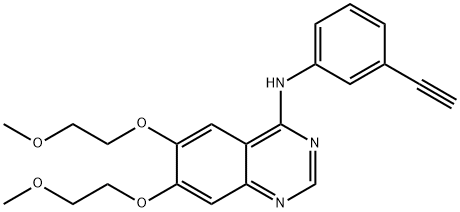
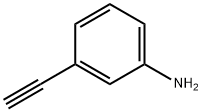
183321-74-6
414 suppliers
$5.00/25mg

183319-69-9
696 suppliers
$5.00/25mg
Yield:183319-69-9 100%
Reaction Conditions:
with hydrogenchloride in isopropyl alcohol at 0; for 1 h;Product distribution / selectivity;Inert atmosphere;
Steps:
1
Example 1 : Erlotinib free base (180 g) containing 15% w/w isopropyl alcohol (IPA) was charged into a three necked flask under nitrogen and cooled to O0C. The solid was exposed to hydrogen chloride gas for about 1 hour to form the hydrochloride salt with stirring. The erlotinib hydrochloride was then dried at 2O0C to 250C in a vacuum oven. The yield was quantitative. 1H NMR (DMSO-d6): £3.36 (s, 6H), 3.79 (m, 4H), 4.28 (s, 1 H), 4.31 (t, J = 4.6 Hz, 2H),4.41 (t, J = 4.8 Hz, 2H), 7.39-7.43 (m, 2H), 7.48 (t, J = 7.9 Hz, IH), 7.82 (d, J = 8.4 Hz, 1 H), 7.90 (s, 1 H), 8.51 (s, 1 H), 8.83 (s, 1 H), 1 1.68 (s, 1 H).
References:
WO2010/40212,2010,A1 Location in patent:Page/Page column 8
54060-30-9
536 suppliers
$8.00/5g

183319-69-9
696 suppliers
$5.00/25mg
![4-Quinazolinamine, 6,7-bis(2-methoxyethoxy)-N-[3-[2-(trimethylsilyl)ethynyl]phenyl]-](/CAS/20210305/GIF/766497-25-0.gif)
766497-25-0
1 suppliers
inquiry

183319-69-9
696 suppliers
$5.00/25mg
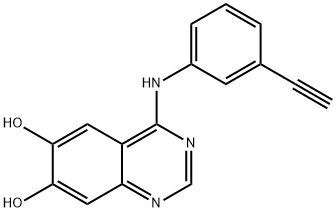
938185-06-9
25 suppliers
inquiry

4296-15-5
59 suppliers
$27.00/100mg

183319-69-9
696 suppliers
$5.00/25mg
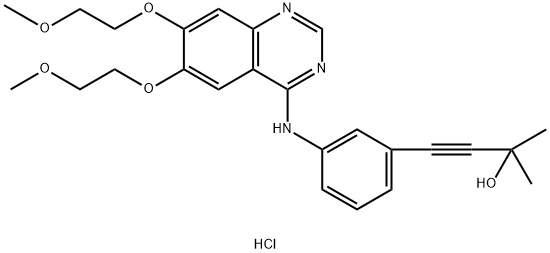
299912-59-7
16 suppliers
inquiry

183319-69-9
696 suppliers
$5.00/25mg