Ivabradine Impurity 1 Hydrochloride synthesis
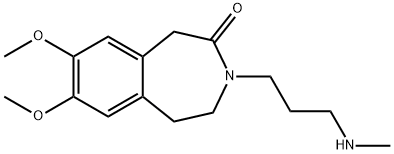
- Product Name:Ivabradine Impurity 1 Hydrochloride
- CAS Number:85175-77-5
- Molecular formula:C16H24N2O3
- Molecular Weight:292.37
Yield:85175-77-5 80%
Reaction Conditions:
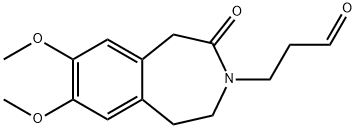
Stage #1: 3-(7,8-dimethoxy-2-oxo-1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)propanal;methylamine in methanol;water at 0; for 1 h;
Stage #2: with methanol;sodium tetrahydroborate in water at 0 - 20; for 12.5 h;
Steps:
1 7,8-dimethoxy-3-[3-(methylamino)propyl]-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one
EXAMPLE 1 7,8-Dimethoxy-3-[3-(methylamino)propyl]-1,3,4,5-tetrahydro-2H-3-benzazepin-2-one Dissolve 50 g (0.18 mol) of 3-(7,8-dimethoxy-2-oxo-1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)propanal in 625 ml of methanol. Cool the resulting solution to 0° C. and then add 62.5 ml (0.81 mol; 4.5 equivalents) of an aqueous 40% methylamine solution. Stir for one hour at 0° C. and then add 7.5 g (0.2 mol; 1.1 equivalent) of NaBH4. Stir for 30 minutes at 0° C. and then stir for 12 hours at ambient temperature. Evaporate off the methanol. The residue is taken up in aqueous hydrochloric acid solution (1N), and washed with ethyl acetate. The aqueous phase is then adjusted to pH=8 by adding 20% sodium hydroxide solution and extracted with dichloromethane. The organic phase is washed with water, dried over MgSO4, filtered and then evaporated to dryness to obtain 52 g of an oil, which is purified by flash chromatography on 1.5 kg of silica (eluant=dichloromethane/-ethanol/NH4OH:80/20/2). 42 g of the expected product are obtained in the form of a white solid. Yield=80% M.p. (KB)=68-70° C.
References:
US2010/160628,2010,A1 Location in patent:Page/Page column 3
![2H-3-Benzazepin-2-one, 1,3-dihydro-7,8-dimethoxy-3-[3-[methyl(phenylmethyl)amino]propyl]-](/CAS/20210305/GIF/96254-71-6.gif)
96254-71-6
0 suppliers
inquiry

85175-77-5
24 suppliers
inquiry
![(Z)-3-(3-chloropropyl)-7,8-diethyl-1H-benzo[d] azepin-2 (3H)-one](/CAS/GIF/85175-59-3.gif)
85175-59-3
244 suppliers
$6.00/250mg

85175-77-5
24 suppliers
inquiry