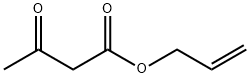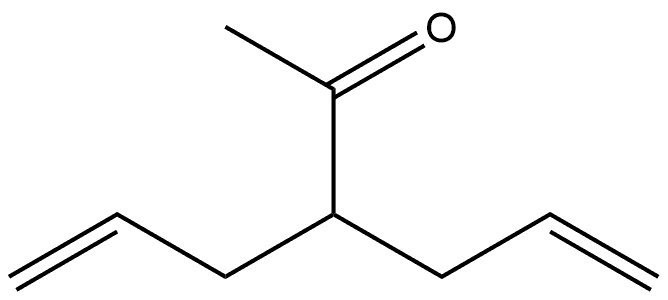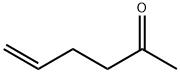
Allylacetone synthesis
- Product Name:Allylacetone
- CAS Number:109-49-9
- Molecular formula:C6H10O
- Molecular Weight:98.14
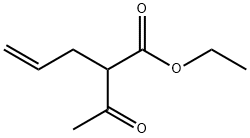
610-89-9
29 suppliers
$60.00/500mg

109-49-9
128 suppliers
$12.00/1g
Yield:109-49-9 80%
Reaction Conditions:
with sodium hydroxide in water for 2 h;Reflux;
Steps:
1 4.3 General procedure for decarbethoxylation of 2-alkenyl β-ketoesters. Preparation of ketones 2a-f
β-Ketoester 1a-f (20 mmol) in aqueous NaOH (2.40 g in 30 mL deionized water) was heated to reflux for 2 h. The crude product was isolated by extraction of the aqueous solution with diethyl ether (3×20 mL). The combined organic layers were dried (Na2SO4) and evaporated. A residue was distilled under reduced pressure. 4.3.1
Hex-5-en-2-one (2a)
Yield 1.57 g, 80%, liquid; IR (ATR) νmax: 3080, 2979, 2921, 1718, 1642, 1431, 970 cm-1; 1H NMR (200 MHz, CDCl3) δ 2.16 (s, 3H), 2.26-2.40 (m, 2H), 2.48-2.59 (m, 2H), 4.94-5.10 (m, 2H), 5.70-5.92 (m, 1H); 13C NMR (50 MHz, CDCl3) δ 27.8, 29.9, 42.7, 115.2, 137.0, 208.1. Anal. Calcd for C6H10O: C: 73.43, H: 10.27%. Found: C: 73.45, H: 10.21%.
References:
?mit, Biljana M.;Pavlovi?, Radoslav Z. [Tetrahedron,2015,vol. 71,# 7,p. 1101 - 1108]
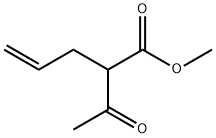
3897-04-9
4 suppliers
inquiry

109-49-9
128 suppliers
$12.00/1g
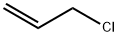
107-05-1
421 suppliers
$16.80/100ML

109-49-9
128 suppliers
$12.00/1g
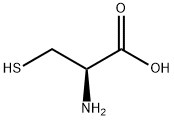
52-90-4
947 suppliers
$6.00/10g
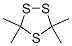
38348-31-1
0 suppliers
inquiry
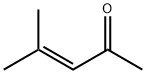
141-79-7
280 suppliers
$10.00/5g

109-49-9
128 suppliers
$12.00/1g
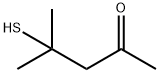
19872-52-7
242 suppliers
$5.00/100mg
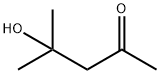
123-42-2
595 suppliers
$19.00/100ml
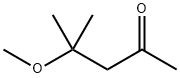
107-70-0
69 suppliers
$35.00/25mL
124-38-9
129 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry