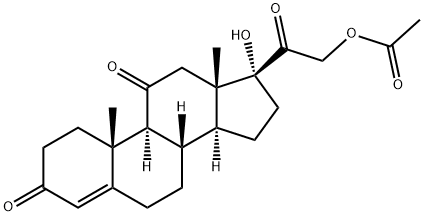
Cortisone acetate synthesis
- Product Name:Cortisone acetate
- CAS Number:50-04-4
- Molecular formula:C23H30O6
- Molecular Weight:402.48

7753-60-8
139 suppliers
$60.00/25 g

50-04-4
352 suppliers
$37.27/1G
Yield:50-04-4 95.8%
Reaction Conditions:
Stage #1:anecortave with tetrafluoroboric acid in dichloromethane;acetone at -5 - 0; for 5 h;Inert atmosphere;
Stage #2: with Jones reagent in dichloromethane;acetone at 0 - 5; for 4 h;Inert atmosphere;Reagent/catalyst;Solvent;Temperature;
Steps:
1-4 Example 4
A, under nitrogen protection, 20ml of acetone, 100ml of dichloromethane, 2ml of 40% fluoroboric acid was added to the reaction flask, cooled to -5-0 ° C, followed by the addition of 20g of anecortave acetate, 10g of dibromohydantoin, heat preservation reaction 5 hours, TLC (dichloromethane: acetone = 6:1) detected no spot on Rf0.91, quenched by adding 15ml of 20% sodium sulfite solution;B. Slowly add 20 ml of Jones reagent to the reaction solution, keep the temperature at 0-5 ° C, and keep the incubation time for about 1 hour. After the addition is completed, the reaction is kept for 3 hours. TLC (dichloromethane: acetone = 6:1) Detecting that there is no spot at Rf0.75, and adding 30 ml of 20% sodium sulfite solution to quench the reaction;C. Add 20 ml of drinking water to the system, stir for 5 minutes, let stand for 30 minutes, separate the organic layer, extract the aqueous layer with 20 ml of chloroform, combine the organic layers, add 10 ml of glacial acetic acid to the organic layer, and control the temperature 5- Add 10 g of zinc powder at 10 ° C, react for 3 hours, detect no spots at Rf 0.86 by TLC (dichloromethane: acetone = 6:1), filter, concentrate the filtrate under reduced pressure, recover the solvent, filter, wash with water until neutral, dry 10h, obtained cortisone acetate (Compound IV) 19.16g, yield 95.8%, HPLC content 99.38%, the largest single impurity 0.21%.
References:
Henan Lihua Pharmaceutical Co., Ltd.;Wang Haibo;Xue Xiao;Chen Yuzhen;Li Hexing CN109942660, 2019, A Location in patent:Paragraph 0012-0015
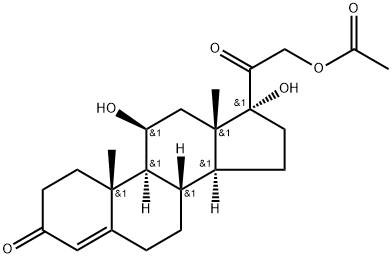
50-03-3
593 suppliers
$32.70/1g

50-04-4
352 suppliers
$37.27/1G
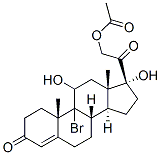
50733-54-5
22 suppliers
inquiry

50-04-4
352 suppliers
$37.27/1G
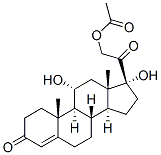
1250-97-1
33 suppliers
$1910.00/250mg

50-04-4
352 suppliers
$37.27/1G
2206-03-3
0 suppliers
inquiry

50-04-4
352 suppliers
$37.27/1G