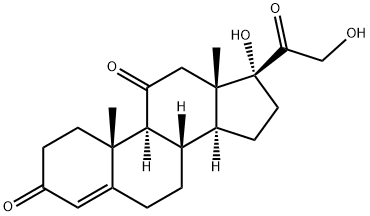
CORTISONE synthesis
- Product Name:CORTISONE
- CAS Number:53-06-5
- Molecular formula:C21H28O5
- Molecular Weight:360.44
53-03-2
411 suppliers
$28.00/1g

53-06-5
223 suppliers
$25.00/250mg
Yield:53-06-5 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrazine hydrate in methanol at 62 - 64; for 8 h;
Steps:
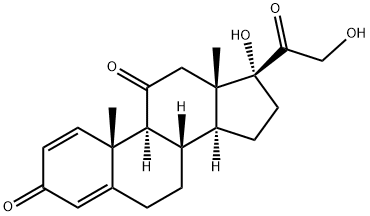
9 Selective reduction of 17α, 21-dihydroxy-4,6-pregnadiene-3,11,20-trione to 17α, 21-dihydroxy-4-pregneno-3,11,20-trione
1] Add 358.4g of 17α, 21-dihydroxy-4,6-pregnadiene-3,11,20-trione, 3600ml of methanol, 60.0g of 10% palladium carbon, 110.0g of hydrazine hydrate to a 5L reactor , Stir to warm to reflux (62 ~ 64 ° C);2] Reaction at reflux (temperature: 62-64 ° C) for 8 hours;3] When the reaction is completed, stop heating and filter off the catalyst while hot;4] The filtrate is heated, and the solvent is distilled under normal pressure;5] Distill off 3200ml of solvent and add 2350ml of purified water;6] Reduce the temperature to 5-20 ° C, suction filter, wash twice with 800ml purified water, and dry to obtain 353.1g of 17α, 21-dihydroxy-4-pregnene-3,11,20-trione crystals with a yield of 98.0 %.
References:
CN104530166,2016,B Location in patent:Paragraph 0098-0105
50-04-4
348 suppliers
$37.27/1G

53-06-5
223 suppliers
$25.00/250mg
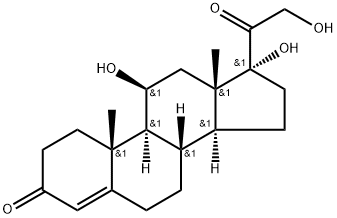
50-23-7
773 suppliers
$24.00/1g

53-06-5
223 suppliers
$25.00/250mg
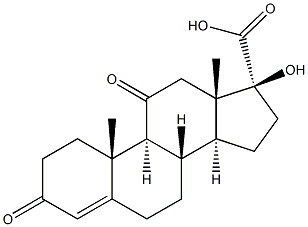
3597-44-2
1 suppliers
inquiry

53-06-5
223 suppliers
$25.00/250mg
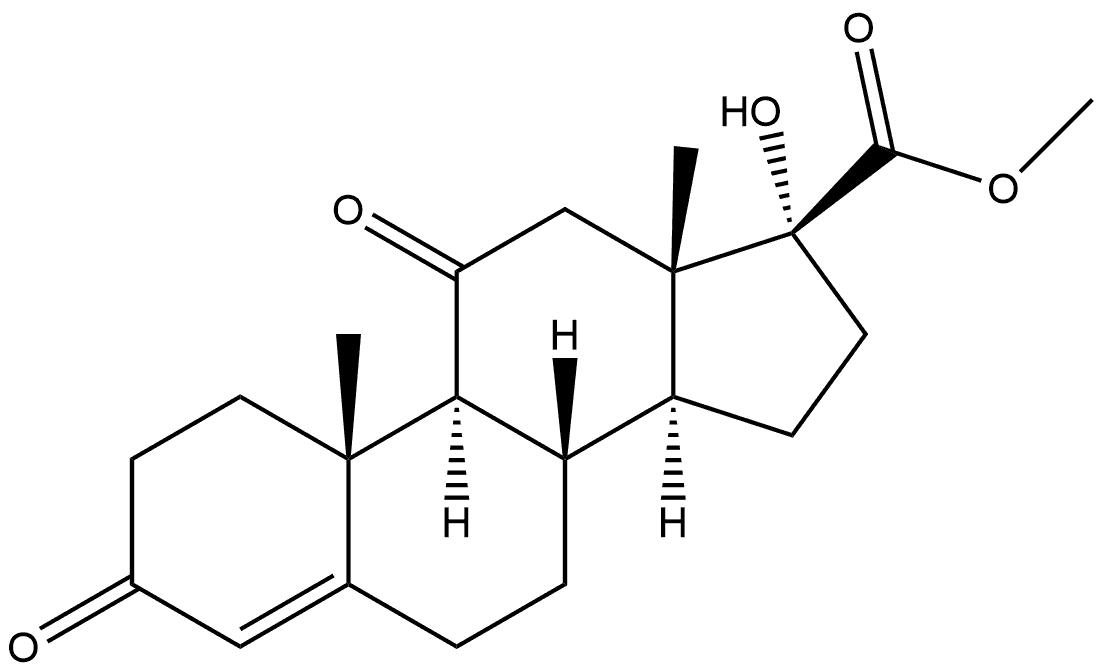
3941-62-6
0 suppliers
inquiry

53-06-5
223 suppliers
$25.00/250mg