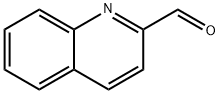
2-Quinolinecarboxaldehyde synthesis
- Product Name:2-Quinolinecarboxaldehyde
- CAS Number:5470-96-2
- Molecular formula:C10H7NO
- Molecular Weight:157.17
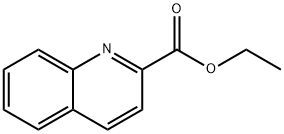
4491-33-2
55 suppliers
$11.00/250mg

5470-96-2
264 suppliers
$7.00/250mg
Yield:5470-96-2 82%
Reaction Conditions:
with diisobutylaluminium hydride in dichloromethane;toluene at -78; for 1.5 h;Inert atmosphere;
Steps:
Quinoline-2-carbaldehyde (7).
To a solution of the quinoline-2-carboxylic acid ethyl ester (6) (1.44 g, 7.17 mmol) in anhydrous CH2Cl2 (20.5 mL) and stirred at -78 °C, under an argon atmosphere, 1M solution in toluene of DIBAL (10.75 mL, 10.75 mmol) was added dropwise, with the aid of a syringe, over a period of 30 minutes. The resulting solution was stirred at the same temperature for a further 1 h. Methanol (6.1 mL) and Rochelle's solution (9.6 mL) were successively added dropwise. Cooling was removed and left to stir overnight. The mixture was filtered with CH2Cl2 by using a pad of celite, which was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude residue was purified by silica gel column chromatography by eluting with hexane/AcOEt (8:2), yielding aldehyde 7 as a yellowish solid in 82% yield. Rf 0.6 (hexane/AcOEt 7:3). mp: 65.3-66.1 °C. IR (neat) νmax/cm-1: 3058, 2933, 2813, 1702, 1264, 1202, 838, 748. 1H NMR (400 MHz, CDCl3) δ 10.23 (s, 1H), 8.29 (d, 1H, J=8.4 Hz), 8.25 (d, 1H, J=8.5 Hz), 8.02 (d, 1H, J=8.4 Hz), 7.89 (d, 1H, J=8.2 Hz), 7.82 (td, 1H, J=7.7, 1.3 Hz), 7.68 (td, 1H, J=7.5, 1.0 Hz). 13C NMR (100 MHz, CDCl3) δ 193.8, 152.6, 147.9, 137.5, 130.6, 130.5, 130.1, 129.3, 128.0, 117.4. HRMS (ESI+) calcd for C10H7NO [M+H]+ 158.0606, found 158.0599.
References:
Diaz-Mu?oz, Gaspar;Isidorio, Raquel Geralda;Miranda, Izabel Luzia;de Souza Dias, Gabriel Nunes;Diaz, Marisa Alves Nogueira [Tetrahedron Letters,2017,vol. 58,# 33,p. 3311 - 3315] Location in patent:supporting information
91-63-4
270 suppliers
$21.60/25g

5470-96-2
264 suppliers
$7.00/250mg
2005-43-8
213 suppliers
$6.00/250mg
201230-82-2
1 suppliers
inquiry

5470-96-2
264 suppliers
$7.00/250mg
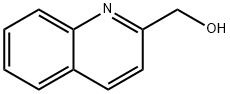
1780-17-2
125 suppliers
$39.00/250mg

5470-96-2
264 suppliers
$7.00/250mg
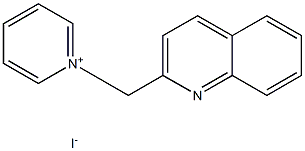
5330-88-1
3 suppliers
inquiry

5470-96-2
264 suppliers
$7.00/250mg