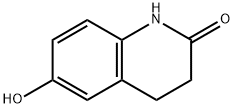
6-Hydroxy-2(1H)-3,4-dihydroquinolinone synthesis
- Product Name:6-Hydroxy-2(1H)-3,4-dihydroquinolinone
- CAS Number:54197-66-9
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
22246-13-5
86 suppliers
$41.00/100mg

54197-66-9
366 suppliers
$8.00/5g
Yield:54197-66-9 216 g
Reaction Conditions:
with sulfuric acid;sodium nitrite in water at 0 - 10; for 0.666667 h;
Steps:
1
15.1 g of compound II and 30.4 Ml of H2SO4 (78%) were placed in a 250 mL reaction flask, stirred, and cooled to 0 to 5 ° C with an ice salt bath, and 31.5 mL of H 2 SO 4 (78%) and 9.2 mL (01186 mol) of HNO 3 (65) were added dropwise. %) of the mixture, control the drop accelerationDegree, keep the reaction temperature below 10 °C, remove the ice bath after the addition is completed, continue to react for 3h, pour the reaction solution into ice water, let it stand, filter it by suction, wash it twice with water, dry the filter cake, and use acetone Crystallization gave 17.6 g of pale yellow solid product 6-nitro-3,4-dihydro-2(1H)quinolinone (III) in a yield of 8912%; mp 203-204 ° C; The peak of 192 is consistent with the mass of the product, and the peaks of each fragment are also consistent;First, 7.63 g of reduced iron powder and 2.75 mL of concentrated hydrochloric acid (36%) were added to the reaction flask, stirred and 150 mL of ethanol (95%) was added, and then the temperature was raised to reflux. After cooling, 7.9 g of the compound was added in portions.III, reheating to reflux, after 2.5h reaction, stop the reaction, heat filtration, and rinse the iron mud in the bottle with hot ethanol for 2 to 3 times, concentrate the filtrate, add a small amount of water, and put it in the refrigerator to cool, a large amount of solids are precipitated. Then, suction filtration, the obtained filter cake was dried to obtain 618 g of pale yellow crystal 6-amino-3,4-dihydro-2(1H)quinolinone (IV), yield 9916%, mp 174-175 ° C; The mass spectrum obtained by mass spectrometry has a peak of 162 which is consistent with the mass of the product, and the peaks of the fragments are also consistent;Dissolve 1.4 g of sodium nitrite in 3.71 mL of water; then pour 4.3 mL of concentrated sulfuric acid (98%) and 617 mL of water into the reaction flask, add 2.95 of the starting compound IV with stirring, stir until it is a paste, and cool with an ice salt bath until 0 to 5 ° C, add a pre-formed aqueous solution of sodium nitrite, control the dropAcceleration, so that the reaction temperature does not exceed 10 ° C; after the addition is completed, the ice bath is removed, heated to reflux with a preheated oil bath, the reaction is stopped for 40 min, the reaction is stopped, 10 mL of water is added, cooled, suction filtered, dried to give 216 g shallow Yellow solid 1, yield 86.7
References:
Panjin Ge Linkaimo Technology Co., Ltd.;Gong Ningrui CN109810054, 2019, A Location in patent:Paragraph 0009; 0010
19313-87-2
49 suppliers
$150.00/1g

54197-66-9
366 suppliers
$8.00/5g
19314-15-9
14 suppliers
$33.39/250mg

54197-66-9
366 suppliers
$8.00/5g
19314-10-4
28 suppliers
inquiry

54197-66-9
366 suppliers
$8.00/5g

7446-70-0
701 suppliers
$15.00/25g

19313-87-2
49 suppliers
$150.00/1g

54197-66-9
366 suppliers
$8.00/5g
