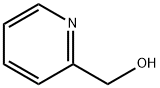
2-(Hydroxymethyl)pyridine synthesis
- Product Name:2-(Hydroxymethyl)pyridine
- CAS Number:586-98-1
- Molecular formula:C6H7NO
- Molecular Weight:109.13
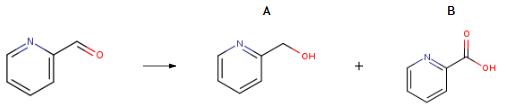
2524-52-9
296 suppliers
$6.00/5g

586-98-1
422 suppliers
$6.00/10g
Yield:586-98-1 99%
Reaction Conditions:
with C30H34Cl2N2P2Ru;potassium methanolate;hydrogen in tetrahydrofuran at 100; under 38002.6 - 76005.1 Torr; for 24 h;Glovebox;Autoclave;
Steps:
27 Example 27: Hydrogenation of ester compounds catalyzed by ruthenium complex Ia
General procedure: In a glove box, add a ruthenium complex Ia (0.3 to 0.7 mg, 0.0002 to 0.001 mmol) to a 300 mL autoclave,Potassium methoxide (35-700 mg, 0.5-10 mmol), tetrahydrofuran (4-60 mL), and ester compounds (10-200 mmol).After sealing the autoclave, take it out of the glove box and fill it with 50 100atm of hydrogen.The reaction kettle was heated and stirred in an oil bath at 100 ° C for 10 to 336 hours.After the reaction kettle was cooled in an ice-water bath for 1.5 hours, the excess hydrogen was slowly released.The solvent was removed from the reaction solution under reduced pressure, and the residue was purified with a short silica gel column to obtain an alcohol compound. The results are shown in Table 5.
References:
CN110357923,2019,A Location in patent:Paragraph 0301-0303; 0306
2459-07-6
242 suppliers
$5.00/1g

586-98-1
422 suppliers
$6.00/10g
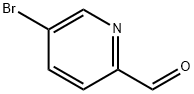
31181-90-5
344 suppliers
$5.00/250mg

586-98-1
422 suppliers
$6.00/10g

1007-49-4
29 suppliers
inquiry

586-98-1
422 suppliers
$6.00/10g
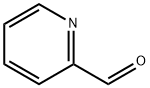
1121-60-4
522 suppliers
$10.60/10gm:

586-98-1
422 suppliers
$6.00/10g