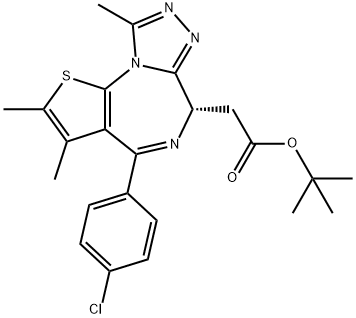
(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate synthesis
- Product Name:(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate
- CAS Number:1268524-70-4
- Molecular formula:C23H25ClN4O2S
- Molecular Weight:456.99
![1H-Thieno[2,3-e]-1,4-diazepine-3-acetic acid, 5-(4-chlorophenyl)-2,3-dihydro-6,7-diMethyl-2-oxo-, 1,1-diMethylethyl ester, (3S)-](/CAS/20150408/GIF/1268524-67-9.gif)
1268524-67-9
24 suppliers
inquiry
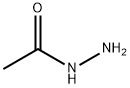
1068-57-1
395 suppliers
$6.00/5g
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](/CAS/GIF/1268524-70-4.gif)
1268524-70-4
239 suppliers
$39.00/1mg
Yield:1268524-70-4 92%
Reaction Conditions:
Stage #1:tert-butyl (S)-2-(5-(4-chlorophenyl)-6,7-dimethyl-2-oxo-2,3-dihydro-1H-thieno[2,3-e][1,4]diazepin-3-yl)acetate with potassium tert-butylate in tetrahydrofuran at -78 - 23; for 0.5 h;
Stage #2: with diethyl chlorophosphate in tetrahydrofuran at -10; for 0.75 h;
Stage #3:acetic acid hydrazide in tetrahydrofuran;butan-1-ol at 23 - 90; for 2 h;
Steps:
Potassium tert-butoxide (1.0 M solution in THF, 0.3 ml, 0.30 mmol, 1.10 equiv) was added to a solution of S5 (114 mg, 0.27 mmol, 1 equiv) in THF (1.8 ml, 0.15 M) at -78 °C. The reaction mixture was warmed to -10 °C, and stirred at 23 °C for 30 min. The reaction mixture was cooled to -78 °C. Diethyl chlorophosphate (0.047 ml, 0.32 mmol, 1.20 equiv) was added to reaction mixture 22. The resulting mixture was warmed to -10 °C over 45 min. Acetic hydrazide (30 mg, 0.40 mmol, 1.50 equiv) was added to reaction mixture. The reaction mixture was stirred at 23 °C. After 1 h, 1-butanol (2.25 ml) was added to reaction mixture, which was heated to 90 °C. After 1 h, all solvents were removed under reduce pressure. The residue was purified with flash column chromatography (Combiflash system, 4 g silica gel, gradient 0 to 100 % ethyl acetate-hexanes) to afford (+)-JQl (114 mg, 92 %) as white solid with 90 % enantiomeric purity (determined with Berger Supercritical Fluid Chromatography (SFC) using AS-H column, 85 % hexanes- methanol, 210 nm, tR (R- enantiomer) = 1.59 min, tR (S-enantiomer) = 3.67 min). The product was further purified by chiral preparative HPLC (Agilent High Pressure Liquid Chromatography using an OD-H column) to provide the S-enantiomer in greater than 99 % ee.1H NMR (600 MHz, CDC13, 25 °C) δ 7.39 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H),4.54 (t, J = 6.6 MHz, 1H), 3.54-3.52 (m, 2H), 2.66 (s, 3H), 2.39 (s, 3H), 1.67 (s, 3H), 1.48 (s, 9H).13C NMR (150 MHz, CDC13, 25 °C) δ 171.0, 163.8, 155.7, 150.0, 136.9, 131.1, 130.9, 130.6, 130.3, 128.9, 81.2, 54.1, 38.1, 28.4, 14.6, 13.5, 12.1.HRMS(ESI) calc'd for C2iH24ClN203S [M+H]+: 457.1460, found 457.1451 m/z.TLC (EtOAc), Rf: 0.32 (UV)[a]22D = + 75 (c 0.5, CHCl3)
References:
DANA-FARBER CANCER INSTITUTE, INC.;BRADNER, James, Elliott;MATZUK, Martin;QI, Jun WO2011/143657, 2011, A1 Location in patent:Page/Page column 82-83
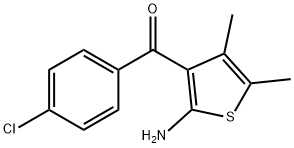
50508-66-2
80 suppliers
$17.00/250mg
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](/CAS/GIF/1268524-70-4.gif)
1268524-70-4
239 suppliers
$39.00/1mg
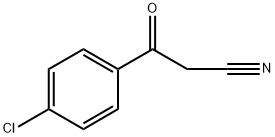
4640-66-8
252 suppliers
$6.00/1g
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](/CAS/GIF/1268524-70-4.gif)
1268524-70-4
239 suppliers
$39.00/1mg
![Butanoic acid, 4-[[3-(4-chlorobenzoyl)-4,5-diMethyl-2-thienyl]aMino]-3-[[(9H-fluoren-9-ylMethoxy)carbonyl]aMino]-4-oxo-, 1,1-diMethylethyl ester, (3S)-](/CAS/20150408/GIF/1268524-65-7.gif)
1268524-65-7
5 suppliers
inquiry
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](/CAS/GIF/1268524-70-4.gif)
1268524-70-4
239 suppliers
$39.00/1mg
![Butanoic acid, 3-aMino-4-[[3-(4-chlorobenzoyl)-4,5-diMethyl-2-thienyl]aMino]-4-oxo-, 1,1-diMethylethyl ester, (3S)-](/CAS/20150408/GIF/1268524-66-8.gif)
1268524-66-8
7 suppliers
inquiry
![(S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate](/CAS/GIF/1268524-70-4.gif)
1268524-70-4
239 suppliers
$39.00/1mg
