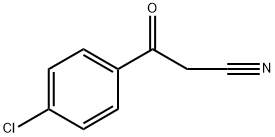
4-CHLOROBENZOYLACETONITRILE synthesis
- Product Name:4-CHLOROBENZOYLACETONITRILE
- CAS Number:4640-66-8
- Molecular formula:C9H6ClNO
- Molecular Weight:179.6
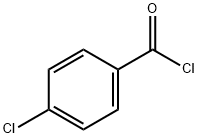
122-01-0
384 suppliers
$13.00/25g
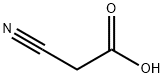
372-09-8
376 suppliers
$20.00/25g

4640-66-8
255 suppliers
$6.00/1g
Yield:4640-66-8 80%
Reaction Conditions:
Stage #1:cyanoacetic acid with [2,2]bipyridinyl;n-butyllithium;magnesium sulfate in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2:4-chloro-benzoyl chloride in tetrahydrofuran;methanol;hexane at -78 - 20;Inert atmosphere;
Stage #3: with hydrogenchloride in tetrahydrofuran;methanol;hexane;water
Steps:
Synthesis of β-keto nitriles
General procedure: Cyanoacetic acid (1.7 g, 20 mmol, 2 equiv), 0.2 mg MgSO4, and ~1 mg 2,2'-bipyridyl was dissolved in tetrahydrofuran (100 mL) and placed in a 500 mL three-neck flask fitted with two dropping funnels and a mechanical stirrer. The system was flushed with nitrogen and cooled to -78 C with a dry ice/ acetone bath. An n-butyl lithium solution (25 mL, 1.6 M in hexanes; 40 mmol, 4 equiv) was added via a dropping funnel with stirring. Once the solution turned slightly purple it was stirred (30 min) after which the acid chloride (10 mmol, 1 equiv) in 5 mL of methanol was added drop-wise with stirring. During this process, the cloudy solution took on a yellow color. The solution was stirred at -78 C for one hour, then the bath was removed and the reaction was allowed to return to room temperature for one hour. An HCl solution (50 mL, 1M) was added drop-wise. At this point, the reaction became clear, while remaining yellow. Water (25 mL) and CH3Cl (50 mL) were added. The aqueous layer was extracted three times with the same volume of CH3Cl. The combined organic layers were washed with two portions (50 mL) of saturated sodium bicarbonate solution and dried over magnesium sulfate, filtered, and reduced on a rotoevaporator. Samples were purified by flash chromatography 6 Hex : 1 EtOAc resulting in percent yields from 50-80%.
References:
Nowill, Randall W.;Patel, Trisha J.;Beasley, David L.;Alvarez, Jose A.;Jackson III, Elizah;Hizer, Todd J.;Ghiviriga, Ion;Mateer, Scott C.;Feske, Brent D. [Tetrahedron Letters,2011,vol. 52,# 19,p. 2440 - 2442] Location in patent:supporting information; experimental part
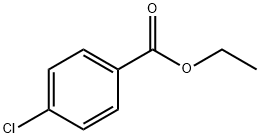
7335-27-5
133 suppliers
$10.00/1g

75-05-8
1016 suppliers
$10.00/10g

4640-66-8
255 suppliers
$6.00/1g
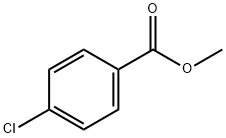
1126-46-1
237 suppliers
$8.00/25g

75-05-8
1016 suppliers
$10.00/10g

4640-66-8
255 suppliers
$6.00/1g
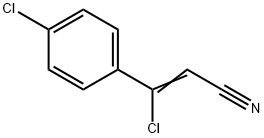
78583-86-5
37 suppliers
$83.50/1g

4640-66-8
255 suppliers
$6.00/1g