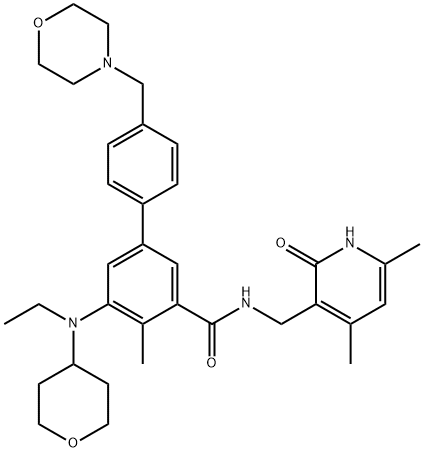
Tazemetostat (EPZ-6438) synthesis
- Product Name:Tazemetostat (EPZ-6438)
- CAS Number:1403254-99-8
- Molecular formula:C34H44N4O4
- Molecular Weight:572.74
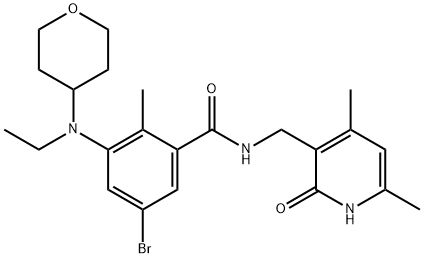
1403257-80-6
31 suppliers
inquiry
![4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)BENZYL]MORPHOLINE](/CAS/GIF/364794-79-6.gif)
364794-79-6
168 suppliers
$6.00/250mg

1403254-99-8
182 suppliers
$25.00/1mg
Yield:1403254-99-8 71%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water;acetonitrile at 100; for 4 h;Inert atmosphere;Suzuki Coupling;
Steps:
44.7
Step 7: Synthesis of N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide To a stirred solution of 5-bromo-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-2-methylbenzamide (14 g, 29.5 mmol) in dioxane/water mixture (70 mL/14 mL) was added 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)morpholine (13.4 g, 44.2 mmol) followed by addition of Na2CO3 (11.2 g, 106.1 mmol). The solution was purged with argon for 15 minutes and then Pd (PPh3)4 (3.40 g, 2.94 mmol) was added and the solution was again purged with argon for a further 10 min. The reaction mixture was heated at 100° C. for 4 h. After completion (monitored by TLC), the reaction mixture was diluted with water and extracted with 10% MeOH/DCM. The combined organic layers were dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure. The crude compound was purified by column chromatography (100-200 mesh silica gel) eluting with methanol: DCM to the title compound as a solid (12 g, 71%). Analytical Data: LCMS: 573.35 (M+1)+; HPLC: 99.5% ( 254 nm) (Rt; 3.999; Method: Column: YMC ODS-A 150 mm*4.6 mm*5μ; Mobile Phase: A; 0.05% TFA in water/B; 0.05% TFA in acetonitrile; Inj. Vol: 10 μL, Col. Temp.: 30° C.; Flow rate: 1.4 mL/min.; Gradient: 5% B to 95% B in 8 min, Hold for 1.5 min, 9.51-12 min 5% B); 1H NMR (DMSO-d6, 400 MHz) δ 11.46 (s, 1H), 8.19 (t, 1H), 7.57 (d, 2H, J=7.2 Hz), 7.36-7.39 (m, 3H), 7.21 (s, 1H), 5.85 (s, 1H), 4.28 (d, 2H, J=2.8 Hz), 3.82 (d, 2H, J=9.6 Hz), 3.57 (bs, 4H), 3.48 (s, 2H), 3.24 (t, 2H, J=10.8 Hz), 3.07-3.09 (m, 2H), 3.01 (m, 1H), 2.36 (m, 4H), 2.24 (s, 3H), 2.20 (s, 3H), 2.10 (s, 3H), 1.64-1.67 (m, 2H), 1.51-1.53 (m, 2H), 0.83 (t, 3H, J=6.4 Hz).
References:
KUNTZ, KEVIN W.;CHESWORTH, RICHARD;DUNCAN, KENNETH W.;KEILHACK, HEIKE;WARHOLIC, NATALIE;KLAUS, CHRISTINE;ZHENG, WANJUN;SEKI, MASASHI;SHIROTORI, SYUJI;KAWANO, SATOSHI US2012/264734, 2012, A1 Location in patent:Page/Page column 128
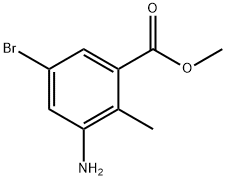
1000342-11-9
180 suppliers
$6.00/1g

1403254-99-8
182 suppliers
$25.00/1mg
![Benzoic acid, 5-broMo-2-Methyl-3-[(tetrahydro-2H-pyran-4-yl)aMino]-, Methyl ester](/CAS/20150408/GIF/1403257-49-7.gif)
1403257-49-7
40 suppliers
$183.33/0.1g

1403254-99-8
182 suppliers
$25.00/1mg
![Benzoic acid, 5-broMo-3-[ethyl(tetrahydro-2H-pyran-4-yl)aMino]-2-Methyl-, Methyl ester](/CAS/20150408/GIF/1403257-79-3.gif)
1403257-79-3
38 suppliers
inquiry

1403254-99-8
182 suppliers
$25.00/1mg
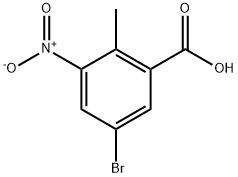
107650-20-4
157 suppliers
$9.00/250mg

1403254-99-8
182 suppliers
$25.00/1mg