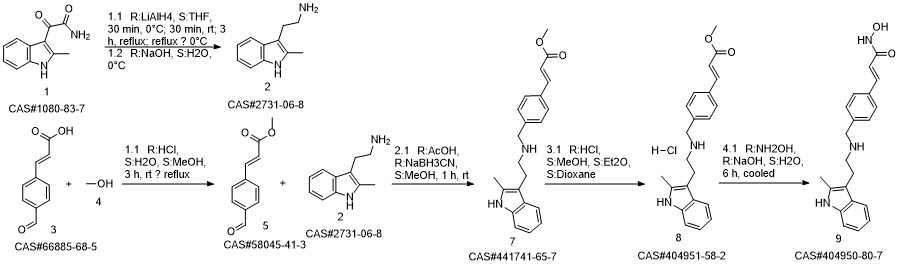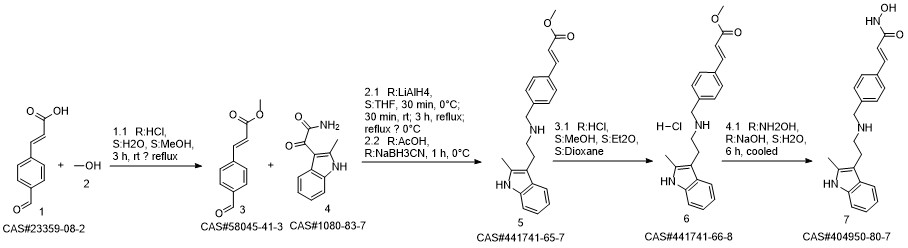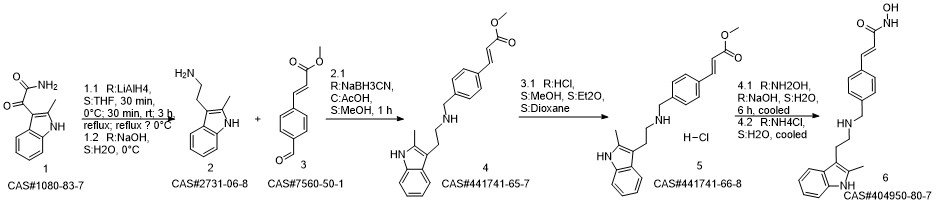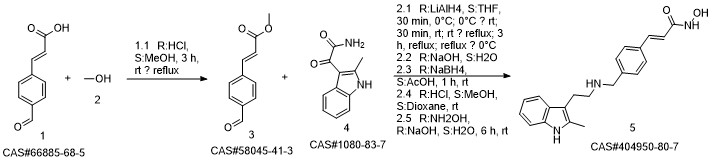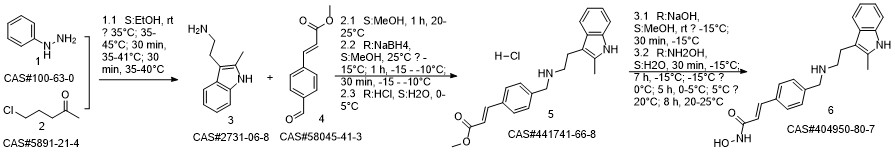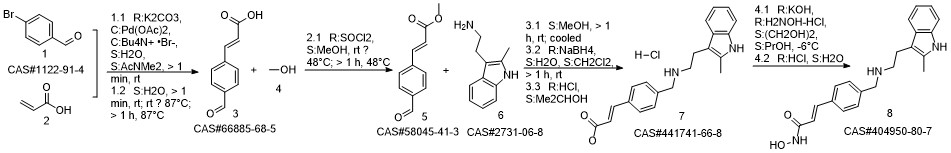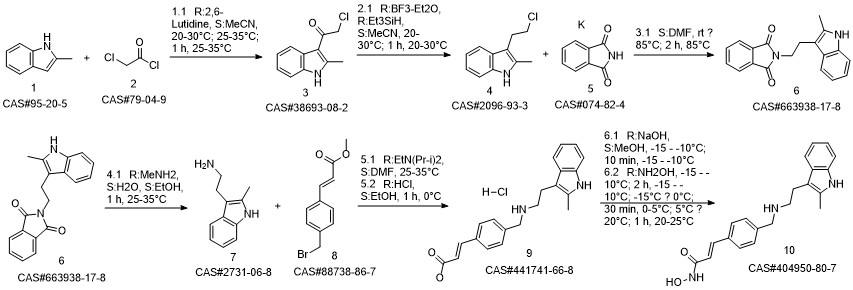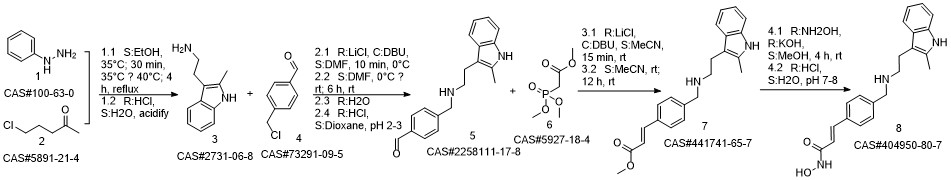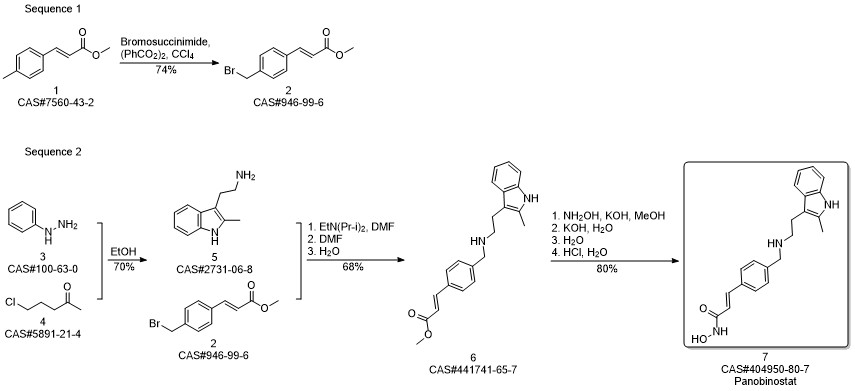
Panobinostat synthesis
- Product Name:Panobinostat
- CAS Number:404950-80-7
- Molecular formula:C21H23N3O2
- Molecular Weight:349.43
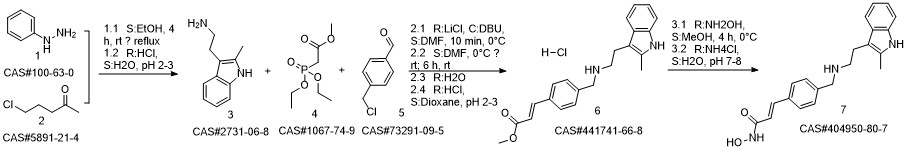
Reference: Chen, Shanwen & Zhang, Peiming & Chen, Huali & Yu, Yu & Gan, Zongjie. (2018). An Improved and Efficient Synthesis of Panobinostat. Journal of Chemical Research. 42. 471-473. 10.3184/174751918X15357309308931.
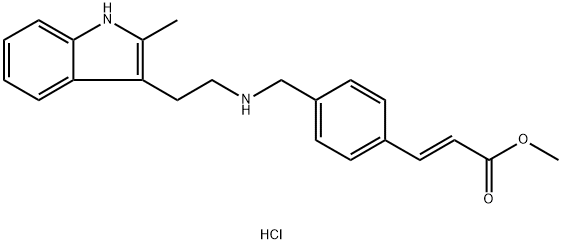
441741-66-8
21 suppliers
$165.00/5mg

404950-80-7
254 suppliers
$28.00/1unit
Yield:404950-80-7 94.6%
Reaction Conditions:
Stage #1:(E)-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]acrylic acid methyl ester hydrochloride with sodium hydroxide in methanol at -15 - -10; for 0.166667 h;
Stage #2: with hydroxylamine hydrochloride in methanol at -15 - -10; for 3.66667 h;
Steps:
11 Embodiment 11(E)- N - hydroxy -3 - [4 - [[ [2 - (2 - methyl - 1H - indole -3 - yl) ethyl] amino] methyl] phenyl] acrylamide
To a 250 ml four-necked flask was added (E) -3- [4 - [[2- (2-methyl-1H-indol-3-yl)(15 g, 0.039 mol), methanol (75 ml), cooled at -10 ° C to 15 ° C with stirring, and rapidly precipitated with sodium hydroxide (4.68 g, 0.71 m 1 ) In methanol solution, drop the mixture, stir lOmin, dropping hydroxylamine solution (32. 52g 50% aqueous solution corresponding to hydroxylamine hydrochloride 16.268,0.234111001), dropping finished at -10 ° (: - 15 ° (temperature insulation Stir for 2 hours.The reaction mixture was warmed to 0 ° C, kept at 0-5 ° C for 30 min, then warmed to 20 ° C, kept at 20-25 ° C for 1 hour, drop in water 38 ml, stir l0 min, Filtered and rinsed with 38 ml of water. The resulting filtrate was adjusted to pH 10 with an aqueous solution of hydrochloric acid (about 18. 5 g of an aqueous solution of 2 mol / L) to 10. The crystals were stirred at 20-25 ° C and treated with hydrochloric acid (About 15.5 g of 2 mol / L of aqueous solution), continue to adjust the PH value of the solution to 8-9, stirring at 20-25 ° C for 1 hour, continue to adjust the pH of the solution to 3-4, stirring for 1 hour , The solid was filtered and the filter cake was rinsed with 50 ml of a methanolic water mixture (ν / ν = 1: 1) and dried to a constant weight in a 50 ° hot air oven to give a solid 12. 89 g, yield 94. 6% :
References:
Nanjing Kawendixu Biological Engineering Co., Ltd.;Xu Yongxiang CN106674079, 2017, A Location in patent:Paragraph 0089; 0090; 0091
![2-Propenoic acid, 3-[4-[[[2-(2-Methyl-1H-indol-3-yl)ethyl]aMino]Methyl]phenyl]-, Methyl ester, (2E)-](/CAS/GIF/441741-65-7.gif)
441741-65-7
16 suppliers
inquiry

404950-80-7
254 suppliers
$28.00/1unit
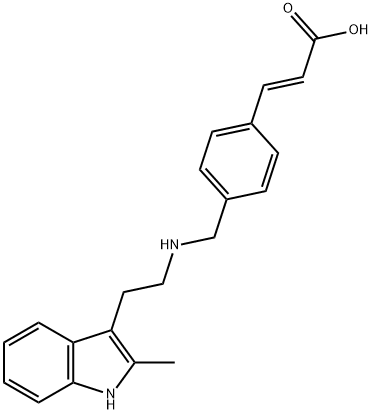
960058-93-9
2 suppliers
inquiry

404950-80-7
254 suppliers
$28.00/1unit
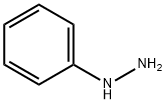
100-63-0
384 suppliers
$10.00/1g

404950-80-7
254 suppliers
$28.00/1unit
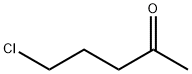
5891-21-4
370 suppliers
$5.00/5g

404950-80-7
254 suppliers
$28.00/1unit