
1,5-Dibromopentane synthesis
- Product Name:1,5-Dibromopentane
- CAS Number:111-24-0
- Molecular formula:C5H10Br2
- Molecular Weight:229.94
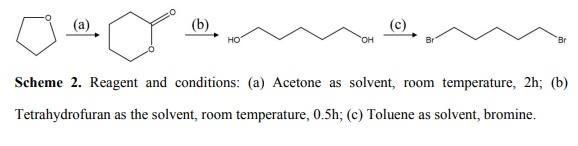
1,5-Dibromopentane synthesis route

111-29-5
395 suppliers
$5.00/25g

111-24-0
411 suppliers
$5.00/5g
Yield:111-24-0 88%
Reaction Conditions:
with hydrogen bromide in octane;water at 148 - 150; for 6 h;Dean-Stark;Temperature;
Steps:
General procedure
General procedure: A one-neck rb flask was charged with diol (1equiv), 48% aq HBr (~3 equiv/OH), octane (~7:1 v/w ratio vs diol), fitted tothe fractionating column/Dean-Stark trap, and heated inan oil bath (145-150 °C) w/rapid magnetic stirring. The aqueous (lower) layer of the initialazeotrope condensate (bp 89-92 °C) was tapped offuntil about half of the theoretical amount of H2O had been collected;the azeotrope temp (still head) then began to rise. The condenser was set to total reflux forseveral h, reopened, and aq material collected for 1h more (head temp 96-100°C). The final volume of aq distillatewas 90-100+% of theory (higher-boiling distillate contained up to 24% HBr). When the (pale tan) octane phase containedboth dibromide and bromoalkanol (6band 6c), washing with cold 85% v/v H2SO4 (10 mL,then 5 mL) removed all color and all bromoalkanol. For all three dibromides (3b, 4b, 6b) the neutralized octane solutionwas stripped of solvent (vigreux column, reduced pressure), and the essentiallypure residue (1H NMR) was kugelrohr distilled. A trace of 4-methyltetrahydropyran was foundin 4b before distillation.
References:
Mekala, Shekar;Hahn, Roger C. [Tetrahedron Letters,2015,vol. 56,# 4,p. 630 - 632] Location in patent:supporting information
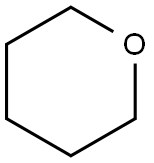
142-68-7
193 suppliers
$13.00/25mL

111-24-0
411 suppliers
$5.00/5g
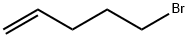
1119-51-3
386 suppliers
$12.00/5g

111-24-0
411 suppliers
$5.00/5g

111-29-5
395 suppliers
$5.00/25g

111-24-0
411 suppliers
$5.00/5g

34626-51-2
183 suppliers
$13.43/250mgs:

