
Dibromomethane synthesis
- Product Name:Dibromomethane
- CAS Number:74-95-3
- Molecular formula:CH2Br2
- Molecular Weight:173.83
6 CH2Cl2 + 3 Br2 + 2 Al → 6 CH2BrCl + 2 AlCl3
CH2Cl2 + HBr → CH2BrCl + HCl
The latter route requires aluminium trichloride as a catalyst. The bromochloromethane product from either reaction can further react in a similar manner:
6 CH2BrCl + 3 Br2 + 2 Al → 6 CH2Br2 + 2 AlCl3
CH2BrCl + HBr → CH2Br2 + HCl
In the laboratory, it is synthesized from bromoform:
CHBr3 + Na3AsO3 + NaOH → CH2Br2 + Na3AsO4 + NaBr
using sodium arsenite and sodium hydroxide.
Another way is to synthesize it from diiodomethane and bromine.

6317-18-6
396 suppliers
$39.00/5g

540-72-7
461 suppliers
$13.50/100G

74-95-3
269 suppliers
$10.00/10g
Yield:74-95-3 79.3%
Reaction Conditions:
Stage #1: bis(thiocyanato)methane;sodium thiocyanide in methanol;water at 74 - 75; for 5 h;
Stage #2: with sodium hydrogencarbonate in methanol;water; pH=6.3;Concentration;Solvent;
Steps:
7 Example 7 Procedure for the preparation of MBT in methanol as solvent (Run 3929Q- 49)
Into a 0.5 L glass reactor equipped with a mechanical stirrer, condenser and thermocouple were introduced water (105 g) and methanol (56 g). Sodium thiocyanate (NaSCN, 150 g, 1.852 mol) was added at 22°C under stirring. After complete dissolution of the NaSCN, dibromomethane (CH2Br2, 155 g, 0.89 mol) was added. The reaction mixture was heated to reflux (74°C) and held at 74-75°C for 5 h, with stirring. Hot water (150 g) was added and the reaction mixture was heated to 84°C over 1.0 h then the heating (Tr = 84-86°C) was continued for 2.0 hours. Methanol (35 g) was added dropwise to the reaction mixture then the reactor was cooled gradually and slowly in order to crystallize the MBT. The reaction mixture was neutrahzed with aq. 9% NaHCOe (25 g) to pH 6.3. The slurry obtained was filtered and washed with methanol/water 20^80 (100 g) and water in two batches (2 x 100 g). The wet product (107.6 g) was dried in an evaporator at 60°C and a vacuum of 30 mm Hg over 5 h. MBT (91.8 g) was obtained in a purity of >99.0% by HPLC, area%. The yield was 79.3%, based on DBM.
References:
WO2014/167560,2014,A1 Location in patent:Page/Page column 14; 15
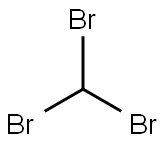
75-25-2
345 suppliers
$16.00/50g

74-95-3
269 suppliers
$10.00/10g
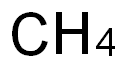
34557-54-5
0 suppliers
inquiry
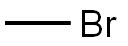
74-83-9
0 suppliers
$28.30/1ML

75-25-2
345 suppliers
$16.00/50g

74-95-3
269 suppliers
$10.00/10g

75-25-2
345 suppliers
$16.00/50g

2857-97-8
444 suppliers
$7.00/25g
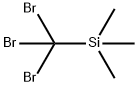
18142-75-1
2 suppliers
inquiry

74-95-3
269 suppliers
$10.00/10g