Ethyl bromoacetate synthesis
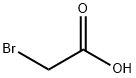
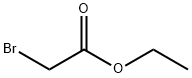
- Product Name:Ethyl bromoacetate
- CAS Number:105-36-2
- Molecular formula:C4H7BrO2
- Molecular Weight:167
Yield:105-36-2 85%
Reaction Conditions:
with sulfuric acid for 24 h;Reflux;
Steps:
A mixture of bromoacetic acid (1.39 g, 10 mmol), sulfuric acid(2 ml, 98%) and ethanol (10 ml, 98%) in a 50 mL round-bottomed flask connected to a reflux condenser, was stirred for 24 h under reflux conditions. Then the mixture was washed with distilled water (3 × 15 ml) to obtain 1.42 g of an oily ethyl bromoacetate with 85% yield. In the next step mixture of ethyl bromoacetate(1.67 g, 10 mmol), was added to a suspension of powdered poly(4-vinylpyridine) (1.05 g) [poly(4-vinylpyridine) cross-linked with 2% DVB -60 mesh, MW: 60,000; Fluka Chemica] in over a period of 5 min. Then, diethyl ether (30 ml) was added to desire product in a round-bottomed flask (50 ml) connected to a reflux condenser and stirred for 24 h under reflux conditions. After removing sol-vent the residue was triturated with diethyl ether (3 × 15 ml), and dried at 50 °C for 1 h. Afterwards, hydrobromic acid (1 ml, 48%) and water (2 ml) was added to reaction mixture and stirred for 2 h under reflux conditions. The solvent was removed and the obtained product was triturated with diethyl ether (3 × 15 ml) and dried under powerful vacuum at 50 °C. Finally, acetic acid functionalized poly(4-vinylpyridinum) bromide (APVPB) was obtained as a white powder.
References:
Moosavi-Zare, Ahmad Reza;Zolfigol, Mohammad Ali;Noroozizadeh, Ehsan;Zarei, Mahmoud;Karamian, Roya;Asadbegy, Mostafa [Journal of Molecular Catalysis A: Chemical,2016,vol. 425,p. 217 - 228]
617-35-6
559 suppliers
$5.00/10g

105-36-2
355 suppliers
$5.00/5G
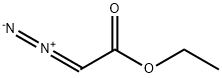
623-73-4
152 suppliers
$27.30/25ML

105-36-2
355 suppliers
$5.00/5G
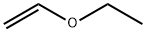
109-92-2
279 suppliers
$17.00/25mL

105-36-2
355 suppliers
$5.00/5G