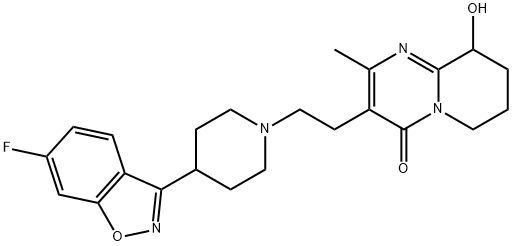
Paliperidone
|
|
Paliperidone ??
- ???
- 158-160°C
- ?? ?
- 612.3±65.0 °C(Predicted)
- ??
- 1.45±0.1 g/cm3(Predicted)
- ???
- 9℃
- ?? ??
- 2-8°C
- ???
- DMSO: ???2mg/mL, ??(??)
- ?? ?? (pKa)
- 13.00±0.60(Predicted)
- ??? ??
- ??
- ??
- ???? ????
- ???
- ??? ?? ?????? 2? ?? ??????. DMSO? ???? -20°?? ?? 2?? ?? ??? ? ????.
- InChI
- InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3
- InChIKey
- PMXMIIMHBWHSKN-UHFFFAOYSA-N
- SMILES
- C12C(O)CCCN1C(=O)C(CCN1CCC(C3C4=C(ON=3)C=C(F)C=C4)CC1)=C(C)N=2
- CAS ??????
- 144598-75-4(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | T,F | ||
|---|---|---|---|
| ?? ???? ?? | 25-39/23/24/25-23/24/25-11 | ||
| ????? | 45-36/37-16 | ||
| ????(UN No.) | UN 2811 6.1/PG 3 | ||
| WGK ?? | 3 | ||
| RTECS ?? | UV1164720 | ||
| ?? ?? | 6.1 | ||
| ???? | III | ||
| HS ?? | 29349990 | ||
| ?? ?? ??? | 144598-75-4(Hazardous Substances Data) | ||
| ?? | rat,LD50,oral,65mg/kg (65mg/kg),Drug Development Research. Vol. 33, Pg. 399, 1994. |
Paliperidone C??? ??, ??, ??
??
Paliperidone, the C-9 hydroxylated active metabolite of the antipsychotic agent risperidone, is the newest atypical antipsychotic to join the market following the introductions of olanzapine (ZyprexaTM), risperidone (RisperdalTM), quetiapine(SeroquelTM), and ziprasidone (GeodonTM). Compared to its parent, paliperidone has improved PK properties and a reduced potential for drug interactions. In terms of receptor affinity, the two drugs are equipotent.??? ??
Off White to Light Orange Coloured Solid??
Paliperidone(Invega) is an atypical antipsychotic. Chemically, paliperidone is the primary active metabolite of the older atypical antipsychotic risperidone. It is indicated for the acute and maintenance treatment of schizophrenia?? ??
Paliperidone, (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one(Invega), is essentially insoluble in water and is available asextended-release tablets. Paliperidone is delivered at a constantrate using an osmotic drug release device (OsmoticRelease Oral Systems [OROS]). The absolute bioavailabilityof paliperidone is 28%, and studies in healthy subjects on ahigh-fat, high-calorie meal showed an increase in AUC.Paliperidone is 74% bound to plasma proteins. After a single,1-mg dose of C-paliperidone, 59% of the dose was excretedin the urine as unchanged drug, and 32% of the dose was recoveredas metabolites. Most of the drug (80%) is excreted bythe kidneys. Paliperidone is metabolized by dealkylation, hydroxylation,dehydrogenation, and scission of the benzoxazolering. None of these metabolic pathways account for morethan 10% of the dose. The terminal elimination half-life ofpaliperidone is 23 hours.???
The most common adverse events included tachycardia, QTc prolongation, headache, anxiety, dizziness, tremors, and insomnia along with the dose-related events of somnolence, orthostatic hypotension, salivary hypersecretion, and extrapyrimidal disorder. Weight gain was also observed in 6–9% of patients which may be attributable to paliperidone’s lower affinity for H1-histaminergic and a1- and a2-adrenergic receptors. Patients with renal impairment require dose adjustments since elimination of paliperidone is altered. Paliperidone is contraindicated in patients with a hypersensitivity to risperidone. Concomitant use of class III antiarrhythmic agents should be avoided since this may result in additive QT interval prolongation. Also, loss of levodopa efficacy is expected with this D2 antagonist.?? ??
1) Beijsterveldt?et al.?(1994),?Regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat; Psychopharmacology,?114?53 DOI:10.1007/BF022454442) Leysen?et al. (1994),?Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity; J. Clin. Psychiatry,?55?suppl: 5 PMID: 7520908
3) Schotte?et al.?(1996),?Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding; Psychopharmacology (Berl.),?124?57 DOI:10.1007/BF02245606
Paliperidone ?? ?? ? ???
???
?? ??
Paliperidone ?? ??
???( 569)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shandong Risen-Sun Pharmaceutical Co., Ltd | +86-15552509998 +86-15621883869 |
liutf@jewim.com.cn | China | 251 | 58 |
| Aceschem Inc. | +1-817863-6948 +1-(817)863-6948 |
sales@aceschem.com | United States | 19632 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8615965530500 |
nickzhang@hangyubiotech.com | China | 10983 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 987 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 |
linda@tnjone.com | China | 1143 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 |
sales@sjar-tech.com | China | 485 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 3237 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3009 | 60 |
Paliperidone ?? ??:
?????? 1-????????-1,1-?????? ????? 2,2,6,6-???-4-????? ????? ??????
Risperidone Impurity 2
9-Keto Risperidone
Paliperidone Palmitate
Paliperidone N-Oxide
Paliperidone Palmitate N-Oxide
Oxiracetam
ILOPERIDONE
(2-Methoxyethyl)benzene
2,2,6,6-Tetramethylpiperidinooxy
4-Ethyl-5-fluoro-6-hydroxypyrimidine
6-Fluoro-3-(4-piperidinyl)benzisoxazole hydrochloride (Paliperidone)
3-(2-Chloroethyl)-2-methyl-9-benzyloxy-4H-pyrido[1,2-a]pyrimidin-4-one (Paliperidone)