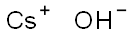Caesiumhydroxid
|
|
Caesiumhydroxid Eigenschaften
- Schmelzpunkt:
- 272 °C(lit.)
- Dichte
- 3.68 g/mL at 25 °C(lit.)
- L?slichkeit
- Miscible with ethanol.
- Aggregatzustand
- Liquid
- pka
- 11.6[at 20 ℃]
- Farbe
- Colorless
- Wasserl?slichkeit
- soluble
- Sensitive
- Air Sensitive
- Merck
- 13,2022
- Expositionsgrenzwerte
- ACGIH: TWA 2 mg/m3
NIOSH: TWA 2 mg/m3
- Stabilit?t:
- Stable. Incompatible with acids. Absorbs carbon dioxide from the air.
- CAS Datenbank
- 21351-79-1(CAS DataBase Reference)
- NIST chemische Informationen
- Cesium hydroxide(21351-79-1)
- EPA chemische Informationen
- Cesium hydroxide (21351-79-1)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | C | ||
|---|---|---|---|
| R-S?tze: | 22-35-34 | ||
| S-S?tze: | 26-36/37/39-45-37/39-27-28 | ||
| RIDADR | UN 2682 8/PG 2 | ||
| OEB | B | ||
| OEL | TWA: 2 mg/m3 | ||
| WGK Germany | 3 | ||
| F | 3 | ||
| TSCA | Yes | ||
| HazardClass | 8 | ||
| PackingGroup | II | ||
| HS Code | 28259090 | ||
| Giftige Stoffe Daten | 21351-79-1(Hazardous Substances Data) | ||
| Toxizit?t | LD50 i.p. in rats: 100 mg/kg (Cochran) |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||
| Sicherheit |
|
Caesiumhydroxid Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE BIS GELBE HYGROSKOPISCHE KRISTALLE.CHEMISCHE GEFAHREN
Reagiert sehr heftig mit Wasser unter Bildung von W?rme. Starke Base. Reagiert sehr heftig mit S?uren. ?tzend auf Metalle. Greift viele Metalle unter Bildung brennbarer/explosionsf?higer Gase an (z.B. Wasserstoff, ICSC-Nr. 0001).ARBEITSPLATZGRENZWERTE
TLV: 2 mg/m?(als TWA) (ACGIH 2004).MAK nicht festgelegt (DFG 2006).
AUFNAHMEWEGE
Aufnahme in den K?rper durch Verschlucken.INHALATIONSGEFAHREN
Eine sch?dliche Partikelkonzentration in der Luft kann schnell erreicht werden durch Dispergieren.WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:Der Stoff ist ?tzend auf die Augen, die Haut und die Atemwege. ?tzend beim Verschlucken.
LECKAGE
Pers?nliche Schutzausrüstung: Atemschutzger?t, P2-Filter für sch?dliche Partikel. Chemikalienschutzanzug. Verschüttetes Material in abdichtbaren Plastikbeh?ltern sammeln. Reste mit viel Wasser wegspülen.R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.R35:Verursacht schwere Ver?tzungen.
R34:Verursacht Ver?tzungen.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S27:Beschmutzte, getr?nkte Kleidung sofort ausziehen.
S28:Bei Berührung mit der Haut sofort abwaschen mit viel . . . (vom Hersteller anzugeben).
Chemische Eigenschaften
Cesium hydroxide is a colorless-to-yellow crystalline compound. It is often used in a water solution.Physikalische Eigenschaften
White to yellowish fused crystalline mass; highly deliquescent; very alkaline; density 3.68 g/cm3; melts 272°C; highly soluble in water; soluble in ethanol; aqueous solution is very alkaline.Verwenden
Cesium hydroxide is used in the preparation of porous cesium titanosilicates, which are potential ion-exchange materials utilized for radioactive wastes cleaning purpose. It is also involved in the preparation of other cesium salts. Further, it is used as an electrolyte in alkaline storage batteries and as a catalyst in organic synthesis. In addition to this, it is used in color photography.Definition
ChEBI: Caesium hydroxide is an alkali metal hydroxide and a caesium molecular entity.synthetische
Cesium hydroxide is prepared by electrolysis of cesium salts to obtain cesium metal, which then reacts with water to yield hydroxide. It also is prepared by the action of barium hydroxide with an aqueous solution of cesium sulfate.Application
Cesium hydroxide also exerts unusual effects in organic reactions, such as the controlled alkylation of amines. In this instance, the cesium ion itself is explicitly involved in the reaction. The hydroxide base promotes an alkylation of a primary amine, while the Cs+ ion weakly coordinates to the amine such that further alkylation is inhibited, preventing formation of dialkyl and trialkyl amines.Cesium hydroxide solution is used in industrial catalysis. Cesium acts as apromoter e.g. in the production of polyols. Polyols are important raw materials for the production of polyurethane foams. Their synthesis profits from use of cesium hydroxide as base catalyst. In polyol synthesis isomerization of the starting material propylene oxide is an adverse side reaction eventually leading to chain termination in the polymerization with polyisocyanate. The tendency of polyols to undergo isomerization is minimized when using cesium hydroxide, as isomerization tendency decreases in the order Li > Na > K > Rb > Cs.
Allgemeine Beschreibung
Cesium hydroxide is a colorless to yellow crystalline solid. Harmful to skin and eyes. Used in electric storage batteries.Air & Water Reaktionen
Dilution with water may generate enough heat to cause steaming or spattering.Reaktivit?t anzeigen
CESIUM HYDROXIDE,SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].Hazard
A poison. Skin, eye, and upper respiratory tract irritant.Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Brandgefahr
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Sicherheitsprofil
Poison by intraperitoneal route. Moderately toxic by ingestion. A powerful caustic. A corrosive skin and eye irritant. See also CESIUM.m?gliche Exposition
Cesium hydroxide may be used as a raw material for other cesium salts; such as the chloride which in turn may be used to produce cesium metal. Cesium metal is used in electronic devices.Versand/Shipping
UN2682 Cesium hydroxide, Hazard class: 8; Labels: 8-Corrosive material. UN2681 Cesium hydroxide, solution, Hazard class: 8; Labels: 8-Corrosive materialInkompatibilit?ten
Cesium hydroxide is the strongest base known. Keep away from all acids. It must be stored in silver or platinum and out of contact with air because of its reactivity with glass. CsOH causes the generation of considerable heat in contact with water or moisture. Contact with many organic compounds, many metals (i.e., aluminum, lead, tin, zinc), glass, oxygen, or carbon dioxide causes a violent reaction.Caesiumhydroxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Caesiumhydroxid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 110)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12809 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17365 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7859 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 |
info@dycnchem.com | China | 52849 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 |
dj@sgtlifesciences.com | China | 12373 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24727 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 |
21351-79-1(Caesiumhydroxid)Verwandte Suche:
Dicesiumoxid
Dicertrioxid
Natriumhydroxid
Calciumdihydroxid
Aluminiumhydroxid
Caesiumfluorid
Ammoniak, wssrige Lsung
Dieisentrioxidhydrat
Indiumtrihydroxid
Kupfercyanid
Caesiumbromid
Caesiumcarbonat
Caesiumsulfat
Caesiumnitrat
Zinkhydroxid
Kupferdihydroxid
Magnesiumhydroxid
Caesium
- Cesium hydroxide, 99.9% metals basis, 50 wt. % solution in water
- CESIUM HYDROXIDE, 99%, 50 WT. % SOLUTION IN WATER
- CESIUM HYDROXIDE, 50% AQUEOUS SOLUTION
- Cesium hydroxide, 50 wt% solution in water, for analysis, 99%
- CESIUM HYDROXIDE, 99% 50% AQUEOUS SOLUTION
- CESIUM HYDROXIDE, 99.9% 50% AQUEOUS SOLUTION
- CESIUM HYDROXIDE, 50% AQUEOUS SOLUTION: 99.9% (METALS BASIS)
- Cesium hydroxide,99%,for analysis,50 wt% solution in water
- Cesium hydroxide, anhydrous
- Cesium hydroxide, 99%, 50 WT% solution in water, for analysis
- Cesium hydoxide
- Caesium Hydroxide, 50% Aqueous Solution
- Cesium hydroxide, 50% w/w aq. soln., 99% (metals basis)
- Cesium hydroxide, 50% w/w aq. soln., 99.9% (metals basis)
- Cesium hydroxide, ca 50 wt% aq. soln.
- Cesium Hydroxide, 50% Aqueous
- Caesium Hydroxide, 50% Aqueous Solution 99.9%
- Caesium Hydroxide 99.9%
- CesiuM hydroxide, for analysis, 50 wt% solution in water, (trace Metal basis)
- CesiuM hydroxide, 99.9%, (trace Metal basis), for analysis, 50 wt% solution in water
- CesiuM hydroxide, 50 wt% solution in water, for analysis, 99% 100GR
- CesiuM hydroxide, 50 wt% solution in water, for analysis, 99% 500GR
- Cesium hydroxide
- Cesium hydroxide, for analysis, 50 wt% solution in water, (trace metal basis), 99.9%
- CesiuM hydroxide, 50 wt.% solution in H2O
- Cesium hydroxide solution, 50 wt. % in water, 99.9% trace metals basis
- Cesium hydroxide solution, 50 wt. % in water, 99% trace metals basis
- caesiumhydroxide,solution
- CesiuM hydroxide solution 50 wt. % in H2O, 99% trace Metals basis (purity deterMined on Metals basis)
- CesiuM hydroxide solution 50 wt. % in H2O, 99.9% trace Metals basis
- Cesium hydroxide (Cs(OH))
- Cesium hydroxide dimer
- cesiumhydroxide(cs(oh))
- cesiumhydroxidedimer
- Cesium hydroxide - 50% solution in water
- CESIUM HYDROXIDE, 99%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99%, 50% AQUEOUS SOLUTION
- CESIUM HYDROXIDE, 99.9%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99.9%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99.9%, 50% AQUEOUS SOLUTIONCESIUM HYDROXIDE, 99.9%, 50% AQUEOUS SOLUTION
- Cesium hydroxide 21351-79-1
- Caesium hydoxide
- CsOH
- Cesiumhydroxide,50%aqueoussolution(99.9%-Cs)
- Cesium hydroxide 50 wt% solution in water
- Cesium hydroxide
- 21351-79-1
- HCsO
- Inorganic Bases
- Synthetic Reagents
- Inorganic Bases
- Materials Science
- Metal and Ceramic Science
- Synthetic Reagents
- Cesium SaltsChemical Synthesis
- Inorganic Bases
- Metal and Ceramic Science
- Salts
- Synthetic Reagents
- Cesium Salts
- Chemical Synthesis