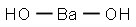Bariumhydroxid
|
|
Bariumhydroxid Eigenschaften
- Schmelzpunkt:
- >300 °C(lit.)
- Siedepunkt:
- decomposes at 1032 (calculated) [KNA91]
- Dichte
- 2.2 g/mL at 25 °C(lit.)
- L?slichkeit
- Aqueous Acid (Slightly), Water (Slightly)
- Aggregatzustand
- Solid
- Farbe
- Clear
- PH
- 11.27(1 mM solution);12.22(10 mM solution);13.08(100 mM solution)
- Geruch (Odor)
- no odor
- Wasserl?slichkeit
- Soluble in water.
- Sensitive
- Air Sensitive & Hygroscopic
- Merck
- 14,977
- Expositionsgrenzwerte
- ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3
- Stabilit?t:
- Stable. Incompatible with acids, carbon dioxide, moisture.
- InChIKey
- RQPZNWPYLFFXCP-UHFFFAOYSA-L
- CAS Datenbank
- 17194-00-2(CAS DataBase Reference)
- NIST chemische Informationen
- Barium dihydroxide(17194-00-2)
- EPA chemische Informationen
- Barium hydroxide (Ba(OH)2) (17194-00-2)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | C,Xi,Xn | ||
|---|---|---|---|
| R-S?tze: | 36/37/38-34-20/22-36/38-41-35-22 | ||
| S-S?tze: | 26-36/37/39-45-36/37 | ||
| RIDADR | UN 3262 8/PG 2 | ||
| WGK Germany | 1 | ||
| RTECS-Nr. | CQ9200000 | ||
| TSCA | Yes | ||
| HazardClass | 8 | ||
| PackingGroup | III | ||
| HS Code | 2805191000 | ||
| Giftige Stoffe Daten | 17194-00-2(Hazardous Substances Data) | ||
| Toxizit?t | LD50 ipr-mus: 255 mg/kg GTPZAB 28(6),45,84 |
| Bildanzeige (GHS) |
 
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | ||||||||||||||
| Gefahrenhinweise |
|
||||||||||||||
| Sicherheit |
|
Bariumhydroxid Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.R34:Verursacht Ver?tzungen.
R20/22:Gesundheitssch?dlich beim Einatmen und Verschlucken.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
Beschreibung
Barium Hydroxide occurs as an anhydrate whose prismatic, colorless crystals are very deliquescent. Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water:BaO+ 9H2O→Ba(OH)2·8H2O
It crystallizes as the octahydrate, which converts to the monohydrate upon heating in air. Barium oxide has been found to react with water vapor at temperatures from 1443 to 1593 K to form gaseous Ba(OH)2 according to the reaction:
BaO(solid)+H2O(gas)→Ba(OH)2(gas)
A better method of preparation involves addition of NaOH to the nitrate. Since barium hydroxide is fairly soluble, it is necessary to evaporate the solution to crystallize Ba(OH)2·8H2O. Ethanol is added to aid in this crystallization. The crystals are washed in cold ethanol to remove sodium ions and then dried.
Chemische Eigenschaften
Barium hydroxide,Ba(OH)2·8H20, is a white crystalline powder, colorless solid composed of monoclinic crystals. It has a melting point of 78°C and is soluble in water and insoluble in acetone. It is used in the fusion of silicates and fat saponification.Physikalische Eigenschaften
Monohydrate, Ba(OH)2?H2O is a white powder; density 3.743 g/cm3; slightly soluble in water; soluble in dilute mineral acids. Octahydrate, Ba(OH)2?8H2O is a colorless monoclinic crystal; density 2.18 g/cm3 at 16°C; refractive index 1.50; melts at 78°C; vapor pressure 227 torr; loses seven molecules of water of crystallization when its solution is boiled in the absence of atmospheric CO2 forming solid monohydrate; further heating produces anhydrous Ba(OH)2 melting at 407°C; readily dissolves in water (3.76 g/100 g at 20°C and 11.7 g/100 g at 50°C); aqueous solution highly alkaline; also soluble in methanol; slightly soluble in ethanol; insoluble in acetone.Verwenden
Barium hydroxide is used in analytical chemistry to titrate weak acids, particularly organic acids. It forms clear aqueous solutions that are free from carbonate, unlike those of the alkali hydroxides, since barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymophthalein (with alkaline color changes) without the risk of titration errors due to the presence of weakly basic CO32- ions.Barium hydroxide is used in organic synthesis as a strong base, for example for the hydrolysis of esters and nitriles.It has been used to hydrolyze one of the two equivalent ester groups in dimethyl endecanedioate. It is also used in the preparation of cyclopentanone, diacetone alcohol and d-Gluonic g-lactone.Barium hydroxide is used as an additive in thermoplastics (such as phenolic resins), rayon and PVC stabilizers to improve plastic properties. This material is used as a general-purpose additive for lubricants and greases. Other industrial applications for barium hydroxide include sugar fabrication, manufacturing soaps, fat saponification, fusing of silicates and chemical synthesis of other barium compounds and organic compounds.
Barium hydroxide is offered for sale both as the octahydrate and the monohydrate. It is available in large quantities commercially.
synthetische
Barium hydroxide is made by dissolving barium oxide in hot water. The octahydrate, Ba(OH)2?8H2O, crystallizes upon cooling. It also is prepared by precipitation with caustic soda from an aqueous solution of barium sulfide:BaS + 2NaOH → Ba(OH)2 + Na2S.
Hazard
An acute poison; toxic symptoms are similar to other soluble salts of barium (see Barium).Sicherheitsprofil
A poison by ingestion andintraperitoneal routes. Incompatible with chlorinated rubber.Bariumhydroxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
myo-Inosit
Heat stabilizer for PVC
BARIUM NITRITE
DL-5-Methoxytryptophan
(R)-2-Amino-3-mercapto-3-methylbutanoic acid
Hex-1-en
Cyclopentane-1,1-diacetic acid
1,3-dihydroxy-2-naphthoic acid
Bariumbis(dinonylnaphthalinsulfonat)
Methansulfonsure
Natriumthiocyanat
Orthophosphorsure
(2-Acetoxyethyl)trimethylammoniumiodid
Perchlorsure
SPIRO[4.5]DECANE-7,9-DIONE
D-Glucono-1,5-lacton
Bariumchromat
Bariumoxalat
2-methylquinolin-3-ol
Natriumperchlorat
Glycin
Cyclopentanol
Dimethylhydrogenphosphat
2-Chlorbenzoes?ure
Thioglykols?ure
Diammin[cyclobutan-1,1-dicarboxylato(2-)-O,O']platin
Hydrogenbromid
4-Methylpentan-2-ol
Bariumperoxid
Kaliumperchlorat
DL-Glyceraldehyd
Kupfer-D-gluconat
Cyclopentanon
Isopropylamin
Arginin
Ammoniumperchlorat
Methylbernsteinsure
Bariumhydroxid Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 331)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5887 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 |
sales@simchoicechem.com | China | 254 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29809 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 |
allison@yan-xi.com | China | 5855 | 58 |
17194-00-2(Bariumhydroxid)Verwandte Suche:
Bariumoxid, bei der Kalzinierung von Witherit gewonnen
Aluminiumhydroxid
Bariumdistearat
Ammoniak, wssrige Lsung
Barium
Calciumdihydroxid
Natriumhydroxid
Bariumcarbonat
Bariumsulfat
Bariumnitrat
Bariumchlorid
Cobaltdihydroxid
Palladiumdihydroxid
Barium hydroxide octahydrate
Barium hydroxide, monohydrate, Ba as BaO 78.5-80.5%
Bariumdibenzoat
Tribariumdicitrat
Bariumdiricinoleat
- Barium hydroxide (Ba(OH)2)
- Barium hydroxide, alpha
- bariumdihydroxide
- bariumhydroxide(ba(oh)2)
- Bariumhydroxide,anhydrous
- BARIUM HYDRATE
- BARIUM HYDROXIDE
- BARIUM CHLORIDE 1 G/L
- Barium hydroxide, anhydrous, anhydrous
- BARIUM HYDROXIDE STANDARD SOLUTION 0,05 MOL/L
- BARIUM HYDROXIDE, HIGH PURITY
- BARIUMHYDROXIDEA10618
- Bariumhydroxide,anhydrous,min.95%
- baryta hydrate
- BARIUM HYDROXIDE-8-HYDRATE TECHNICAL
- BARIUM HYDROXIDE, TECH., CA. 95%
- BARIUM HYDROXIDE STANDARD SOLUTION
- BARIUM HYDROXIDE-8-HYDRATE R. G., REAG.ISO, REAG. PH. EU
- BARIUM HYDROXIDE 0,05 MOL/L VOLUME-TRIC SOLUTION
- BARIUM HYDROXIDE, ANHYDROUS, 94-98%
- BariumHydroxideOctohydrate
- Barium hydroxide, anhydrous, min. 95%
- Barium dihydoxide
- Aetzbaryt
- Barium hydroxide lime
- Barium(2+) hydroxide
- Bariumhydroxid
- Chebi:32592
- Barium Hydroxide, Anhydrous, Powder
- BariuM Hydroxide, Anhydrous, Powder, Reagent
- BariuM hydroxide technical grade, ~95%
- Barium hydroxide test solution(ChP)
- Barium hydroxide ISO 9001:2015 REACH
- Barium hydroxide test solution
- Ba(OH)2
- Anhydrous Barium hydroxide
- A barium dihydroxide test
- Barium hydroxide solution 0.3 N
- 17194-00-2
- H2BaO2
- BaO2H2
- Synthetic Reagents
- Inorganic Bases
- metal hydroxide
- Inorganics
- Barium SaltsChemical Synthesis
- Inorganic Bases
- Metal and Ceramic Science
- Salts
- Synthetic Reagents
- Base SolutionsChemical Synthesis
- By Reference Material
- Inorganic BasesVolumetric Solutions
- Reference Material Hydrochloric acid
- Titration
- Volumetric Solutions
- Chemical Synthesis
- Industrial/Fine Chemicals