| Identification | Back Directory | [Name]
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl | [CAS]
135139-00-3 | [Synonyms]
Xylbinap
(S)-DM-BINAP
RAC-XYLYL-BINAP
98% (S)-XylBINAP
(S)-(-)-XylBINAP
(S)-3,5-Xyl-BINAP
(S)-3,5-XYLYL-BINAP
(R)-3,5-Xylyl-binap ,98%
ethyl 4-oxo-2-(propylamino)furan-3-carboxylate
2,2'-BIS[DI(3,5-XYLYL)PHOSPHINO]-1,1'-BINAPHTHYL
(S)-(-)-2,2'-Bis(di(3,5-xylyl)phosphino)-1,1'-binaphtyl
(S)-(-)-2,2'-BIS[DI(3,5-XYLYL)PHOSPHINO]-1,1'-BINAPHTHYL
RACEMIC-2,2'-BIS[DI(3,5-XYLYL)PHOSPHINO]-1,1'-BINAPHTHYL
4-keto-2-(propylamino)furan-3-carboxylic acid ethyl ester
2,2'-BIS[BIS(3,5-DIMETHYLPHENYL)PHOSPHINO]-1,1'-BINAPHTHYL
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl,98%
(R)-2,2'-Bis(bis(3,5-dimethylphenyl)phosphino)-1,1'-binaphthalene
(S)-2,2'-bis(bis(3,5-dimethylphenyl)phosphanyl)-1,1'-binaphthalene
(S)-(-)-2,2'-bis(di-(3,5-dimethylphenyl)phosphino)-1,1'-binaphthyl
(S)-(-)-2,2'-Bis[bis(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl,(S)-XylBINAP
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl, 98% (S)-(-)-XylBINAP
(S)-(-)-2,2''-BIS(DI(3,5-XYLYL)PHOSPHINO)-1,1''-BINAPHTYL (S)-3,5-XYLYL-BINAP
(S)-(-)-2,2''-BIS(DI(3,5-XYLYL)PHOSPHINO)-1,1''-BINAPHTHYL (S)-3,5-XYLYL-BINAP
(S)-(-)-2,2'-Bis(di-(3,5-dimethylphenyl)phosphino)-1,1'-binaphthyl , ((S)-Xylyl
(S)-(-)-2,2'-Bis[di(3,5-xylyl)phosphino]-1,1'-binaphthyl,98%(S)-3,5-xylyl-BINAP
(S)-(-)-2,2'-Bis(di-(3,5-dimethylphenyl)phosphino)-1,1'-binaphthyl , ((S)-Xylyl-BINAP)
PHOSPHINE, 1,1''-[(1S)-[1,1''-BINAPHTHALENE]-2,2''-DIYL]BIS[1,1-BIS(3,5-DIMETHYLPHENYL)-
(S)-(-)-2,2'-Bis(di-(3,5-dimethylphenyl)phosphino)-1,1'-binaphthyl , 98% ((S)-Xylyl-BINAP)
(S)-3,5-Xylyl-BINAP, (S)-(-)-2,2μ-Bis[di(3,5-xylyl)phoshino]-1,1μ-binaphthyl, (S)-(-)-2,2μ-Bis[bis(3,5-dimethylphenyl)phosphino]-1,1μ-binaphthyl | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C52H48P2 | [MDL Number]
MFCD01630821 | [MOL File]
135139-00-3.mol | [Molecular Weight]
734.89 |
| Chemical Properties | Back Directory | [Melting point ]
203-206°C | [alpha ]
-172 º (c=1 in chloroform) | [Boiling point ]
825.3±65.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [Water Solubility ]
Insoluble in water | [form ]
crystal | [color ]
white to pale yellow | [optical activity]
[α]/D -172°, 20, c =1 in chloroform |
| Questions And Answer | Back Directory | [Reaction]
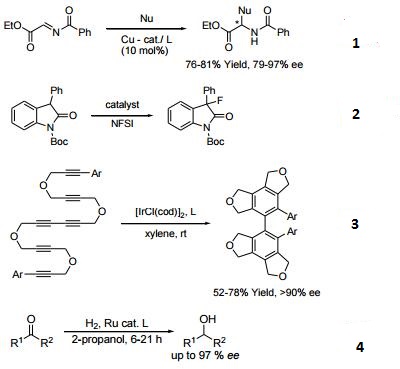
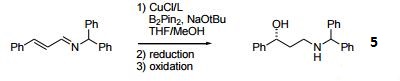
- Ligand used in copper-catalyzed asymmetric Mannich-type reactions of N-acylimino esters.
- Ligand used in the enantioselective fluorination of oxindoles.
- Ligand used in [2+2+2] cycloaddition of tetraynes and hexaynes.
- Ligand used in the asymmetric reduction of ketone via ruthenium-catalyzed transfer hydrogenation.
- Asymmetric hydroboration of unsaturated imines.
|
| Hazard Information | Back Directory | [Uses]
Catalyst for:
- Asymmetric hydrogenation of benzophenone
- Regio- and stereoselective preparation of axially chiral arylnaphthalene derivatives via rhodium-catalyzed [2+2+2] cycloaddition of diynes with naphthalenepropynoic acid derivatives in the presence of chiral biaryl bisphosphine ligands
- Regiodivergent rhodium-catalyzed [(2+2)+2] carbocyclization of 1,6-enynes with Me propiolates
- Ligand controlled regioselective and stereoselective desymmetrizing rhodium-catalyzed allylic arylation of meso cyclopentene dicarbonates with arylboronic acids to form regioisomeric arylcyclopentenols
- Platinum(II) complex-catalyzed enantioselective aldol reaction with ketene silyl acetals in DMF at room temperature
- Stereoselective preparation of chiral N,O-biaryls via Rh-catalyzed [2+2+2] cycloaddition of conjugate ynamides with diynes
|
|
|