| Identification | More | [Name]
Cefazolin | [CAS]
25953-19-9 | [Synonyms]
CEFAZOLIN
CEFAZOLIN ACID
(6r-trans)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-7-[[(1h-tetr
5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,3-(((5-methyl-1,3,4-thia
7-(1-(1h-)-tetrazolylacetamido)-3-[2-(5-methyl-1,3,4-thiadiazolyl)thiomethyl]d
azol-1-yl)acetyl]-amino]-5-thia-1-azabicylo
cefamezin
CefazoilnV
cefazoline
cephamezine
cephazolin
cephazoline
cez
diazol-2-yl)thio)methyl)-8-oxo-7-((1h-tetra
diazol-2-yl)thio)methyl)-8-oxo-7-(2-(1h-t-
diazol-2-yl)thio)methyl)-8-oxo-7-(2-(1h-tetrazol-1-yl)acetamido)-
elta3-cephem-4-carboxylicacid
elzogram
Cephazolin,sodium salt
Cefazoline sodium sterile IP/Bp | [EINECS(EC#)]
247-362-8 | [Molecular Formula]
C14H14N8O4S3 | [MDL Number]
MFCD00243010 | [Molecular Weight]
454.51 | [MOL File]
25953-19-9.mol |
| Chemical Properties | Back Directory | [Melting point ]
198-200 C | [density ]
2.01±0.1 g/cm3(Predicted) | [storage temp. ]
Hygroscopic, -20°C Freezer, Under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Very Slightly, Heated) | [form ]
neat | [pka]
pKa 2.15 (Uncertain) | [color ]
White to Off-White | [Stability:]
Hygroscopic | [CAS DataBase Reference]
25953-19-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3 | [HS Code ]
2941906000 | [Hazardous Substances Data]
25953-19-9(Hazardous Substances Data) | [Toxicity]
mouse,LD50,intramuscular,4gm/kg (4000mg/kg),Byoin Yakugaku. Hospital Pharmacology. Vol. 3, Pg. 220, 1978. |
| Hazard Information | Back Directory | [Description]
Cefazolin has the natural acetyl side chain at C-3 replaced by a thio-linked thiadiazole ring. Although this
group is an activating leaving group, the moiety is not subject to the inactivating host hydrolysis reaction
that characterizes cephapirin. At C-7, it possesses a tetrazoylmethylene unit. Cefazolin is less irritating on
injection than its cohort in this generation of drugs and has a longer half-life than cephapirin. Its dosing
should be reduced in the presence of renal impairment. It is comparatively unstable and should be protected
from heat and light. | [Chemical Properties]
needles | [Originator]
Cefamedin,Fujisawa,Japan,1971 | [Uses]
Antibacterial (systemic). | [Definition]
ChEBI: A cephalosporin compound having [(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl and (1H-tetrazol-1-ylacetyl)amino side-groups. | [Manufacturing Process]
7-Aminocephalosporanic acid is converted to its sodium salt and acylated with
1H-tetrazole-1-acetyl chloride. The acetoxy group is then displaced by
reaction with 5-methyl-1,3,4-thiadiazole-2-thiol in buffer solution. The product
acid is converted to the sodium salt by NaHCO3. | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
Enterobacter, Klebsiella, Providencia, Serratia spp.
and Pr. vulgaris are all resistant. B. fragilis is resistant, but
other anaerobes are susceptible. | [Pharmacokinetics]
Distribution
The volume of distribution is the smallest of the cephalosporins
in group 1, perhaps an indication of relative confinement
to the plasma space. It crosses inflamed synovial
membranes, but the levels achieved are well below those
of the simultaneous serum levels and entry to the CSF is
poor. In patients receiving 10 mg/kg by intravenous bolus,
mean concentrations in cancellous bone were 3.0 mg/kg
when the mean serum concentration was 33 mg/L, giving
a bone:serum ratio of 0.09. Some crosses the placenta,
but the concentrations found in the fetus and membranes
are low.
Metabolism and excretion
It is not metabolized. Around 60% of the dose is excreted
in the urine within the first 6 h, producing concentrations
in excess of 1 g/L. Excretion is depressed by probenecid.
The renal clearance is around 65 mL/min and declines in
renal failure, when the half-life may rise to 40 h, although levels
in the urine sufficient to inhibit most urinary pathogens
are still found. It is moderately well removed by hemodialysis
and less well by peritoneal dialysis.
Levels sufficient to inhibit a number of enteric organisms
likely to infect the biliary tract are found in T-tube bile (17–31
mg/L after a 1 g intravenous dose), but this is principally due
to the high serum levels of the drug and the total amounts
excreted via the bile are small. | [Clinical Use]
Cefazolin has been widely used in surgical prophylaxis,
especially in biliary tract (because of the moderately high
concentrations achieved in bile), orthopedic, cardiac and
gynecological surgery. | [Side effects]
Side effects are those common to other cephalosporins
,including rare bleeding disorders and encephalopathy
in patients in whom impaired excretion or direct instillation
leads to very high CSF levels. Neutropenia has been
described and hypoprothrombinemic bleeding has been
attributed to the side chain. | [Synthesis]
Cefazolin, (6R-trans)-3[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-
7-[(1H-tetrazol-1-ylacetyl)amino]-5-thia-1-azabycyclo[4.2.0]oct-2-en-2-carboxylic acid
(32.1.2.7), is synthesized by reacting 7-aminocephalosporanic acid with a mixed anhy�dride (32.1.2.6), which is the result of a reaction of tetrazolylacetic acid with pivalic
(trimethylacetic) acid chloride. Further reaction with 2-mercapto-5-methyl-1,3,4-thiadia�zole results in a substitution of the 3-acetoxy group with a mercaptothiadiazol group, giv�ing cefazolin (32.1.2.7). 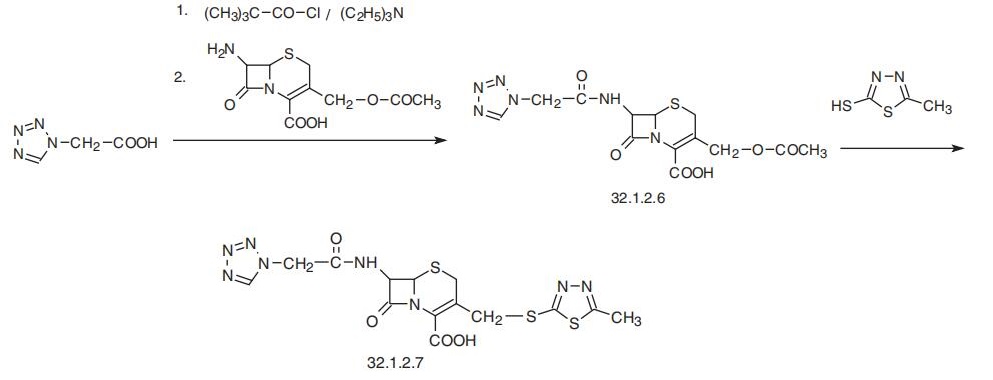
| [storage]
4°C, away from moisture |
|
|