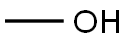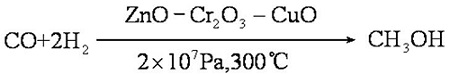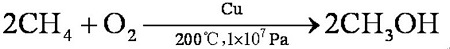| Identification | More | [Name]
Methanol | [CAS]
67-56-1 | [Synonyms]
2-[4-(DIMETHYLAMINO)PHENYLAZO]BENZOIC ACID
4-DIMETHYLAMINOAZOBENZENE-2'-CARBOXYLIC ACID
4-DIMETHYLAMINOAZOBENZENE-2-CARBOXYLIC ACID
ACID RED
ACID RED 2
CI 13020
CI NO 13020
METHYL RED
METHYL RED ETHANOL
METHYL RED INDICATOR
METHYL RED MIXED SOLUTION
METHYL RED MIXED SOLUTION R
METHYL RED, NEUTRAL
METHYL RED SOLUTION R
METHYL RED, SPIRIT SOLUBLE
METHYL RED, WATER SOLUBLE
O-(P-DIMETHYLAMINOPHENYLAZO)BENZOIC ACID
P-DIMETHYLAMINOAZOBENZENE-O-CARBOXYLIC ACID
S NO 250
ai3-00409 | [EINECS(EC#)]
200-659-6 | [Molecular Formula]
CH4O | [MDL Number]
MFCD00002425 | [Molecular Weight]
32.04 | [MOL File]
67-56-1.mol |
| Chemical Properties | Back Directory | [Appearance]
Methyl alcohol is a colorless, volatile liquid
with a mild odor. | [Melting point ]
-98 °C(lit.)
| [Boiling point ]
65.4 °C(lit.)
| [density ]
0.791 g/mL at 25 °C
| [vapor density ]
1.11 (vs air)
| [vapor pressure ]
410 mm Hg ( 50 °C)
| [refractive index ]
n20/D 1.329(lit.)
| [Fp ]
52 °F
| [storage temp. ]
Store at RT. | [solubility ]
benzene: miscible(lit.) | [form ]
Liquid Free From Particulates | [pka]
15.2(at 25℃) | [color ]
<10(APHA)
| [Specific Gravity]
0.793 (20/20℃) | [Flame Color]
Pale blue | [Odor]
Faint alcohol odor detectable at 4 to 6000 ppm (mean = 160 ppm) | [PH]
6.8 (20°C in H2O) | [Relative polarity]
0.762 | [explosive limit]
5.5-44%(V) | [Odor Threshold]
33ppm | [Water Solubility ]
miscible | [λmax]
λ: 210 nm Amax: 0.50
λ: 220 nm Amax: 0.30
λ: 230 nm Amax: 0.15
λ: 235 nm Amax: 0.10
λ: 240 nm Amax: 0.05
λ: 260 nm Amax: 0.01
λ: 400 nm Amax: 0.01 | [Detection Methods]
GC | [Merck ]
14,5957 | [BRN ]
1098229 | [Henry's Law Constant]
4.99 at 25 °C (headspace-GC, Gupta et al., 2000) | [Dielectric constant]
33.6(20℃) | [Exposure limits]
TLV-TWA (200 ppm) (ACGIH), 260mg/m3, 1040mg/m3 (800 ppm) 15minutes (NIOSH); STEL 310mg/m3 (250 ppm); IDLH 25,000 ppm (NIOSH). | [LogP]
-0.770 | [CAS DataBase Reference]
67-56-1(CAS DataBase Reference) | [NIST Chemistry Reference]
Methyl alcohol(67-56-1) | [Storage Precautions]
Moisture sensitive | [EPA Substance Registry System]
67-56-1(EPA Substance) | [Absorption]
in accordance |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,T,F | [Risk Statements ]
R10:Flammable.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R68/20/21/22:Harmful: possible risk of irreversible effects through inhalation, in contact with skin and if swallowed .
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R11:Highly Flammable.
R40:Limited evidence of a carcinogenic effect.
R36:Irritating to the eyes.
R36/38:Irritating to eyes and skin .
R23/25:Toxic by inhalation and if swallowed . | [Safety Statements ]
S36/37:Wear suitable protective clothing and gloves .
S7:Keep container tightly closed .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S16:Keep away from sources of ignition-No smoking .
S24/25:Avoid contact with skin and eyes .
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24:Avoid contact with skin . | [OEB]
A | [OEL]
TWA: 200 ppm (260 mg/m3), STEL: 250 ppm (325 mg/m3) [skin] | [RIDADR ]
UN 1170 3/PG 2
| [WGK Germany ]
1
| [RTECS ]
PC1400000
| [F ]
3-10 | [Autoignition Temperature]
385 °C | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29051100 | [Safety Profile]
A human poison by
ingestion. Poison experimentally by skin
contact. Moderately toxic experimentally by
intravenous and intraperitoneal routes.
Mildly toxic by inhalation. Human systemic
effects: changes in circulation, cough,
dyspnea, headache, lachrymation, nausea or
vomiting, optic nerve neuropathy,
respiratory effects, visual field changes. An
experimental teratogen. Experimental
reproductive effects. An eye and skin
irritant. Human mutation data reported. A
narcotic.
Its main toxic effect is exerted upon the
nervous system, particularly the optic nerves
and possibly the retinae. The condtion can
progress to permanent blindness. Once
absorbed, methanol is only very slowly
eliminated. Coma resulting from massive
exposures may last as long as 2-4 days. In
the body, the products formed by its
oxidation are formaldehyde and formic acid,
both of which are toxic. Because of the slow
elimination, methanol should be regarded as
a cumulative poison. Though single
exposures to fumes may cause no harmful
effect, daily exposure may result in the
accumulation of sufficient methanol in the
body to cause illness. Death from ingestion of less than 30 mL has been reported. A
common air contaminant.
Flammable liquid. Dangerous fire hazard
when exposed to heat, flame, or oxidlzers.
Explosive in the form of vapor when
exposed to heat or flame. Explosive reaction
with chloroform + sodium methoxide,
diethyl zinc. Violent reaction with alkyl
aluminum salts, acetyl bromide, chloroform
+ sodlum hydroxide, CrO3, cyanuric
chloride, (I + ethanol + HgO), Pb(ClO4)2,
HClO4, P2O3, (KOH + CHCb), nitric acid.
Incompatible with berylhum dihydride,
metals (e.g., potassium, magnesium),
oxidants (e.g., barium perchlorate, bromine,
sodium hypochlorite, chlorine, hydrogen
peroxide), potassium tert-butoxide, carbon
tetrachloride + metals (e.g., aluminum,
magnesium, zinc), dlchloromethane.
Dangerous; can react vigorously with
oxidizing materials. To fight fire, use alcohol
foam. When heated to decomposition it
emits acrid smoke and irritating fumes. | [Hazardous Substances Data]
67-56-1(Hazardous Substances Data) | [Toxicity]
LD50 oral (rat)
5628 mg/kg
LD50 skin (rabbit)
15,840 mg/kg
LC50 inhal (rat)
>145,000 ppm (1 h)
PEL (OSHA)
200 ppm (260 mg/m3)
TLV-TWA (ACGIH)
200 ppm (260 mg/m3)—skin
STEL (ACGIH)
250 ppm (328 mg/m3) | [IDLA]
6,000 ppm |
| Hazard Information | Back Directory | [General Description]
A colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products. | [Reactivity Profile]
METHANOL(67-56-1) reacts violently with acetyl bromide [Merck 11th ed. 1989]. Mixtures with concentrated sulfuric acid and concentrated hydrogen peroxide can cause explosions. Reacts with hypochlorous acid either in water solution or mixed water/carbon tetrachloride solution to give methyl hypochlorite, which decomposes in the cold and may explode on exposure to sunlight or heat. Gives the same product with chlorine. Can react explosively with isocyanates under basic conditions. The presence of an inert solvent mitigates this reaction [Wischmeyer 1969]. A violent exothermic reaction occurred between methyl alcohol and bromine in a mixing cylinder [MCA Case History 1863. 1972]. A flask of anhydrous lead perchlorate dissolved in METHANOL(67-56-1) exploded when METHANOL(67-56-1) was disturbed [J. Am. Chem. Soc. 52:2391. 1930]. P4O6 reacts violently with METHANOL(67-56-1). (Thorpe, T. E. et al., J. Chem. Soc., 1890, 57, 569-573). Ethanol or METHANOL(67-56-1) can ignite on contact with a platinum-black catalyst. (Urben 1794). | [Air & Water Reactions]
Highly flammable. Soluble in water in all proportions. | [Hazard]
Flammable, dangerous fire risk. Explosive
limits in air 6–36.5% by volume. Toxic by ingestion
(causes blindness). Headache, eye damage, dizziness, and nausea. | [Health Hazard]
Exposure to excessive vapor causes eye irritation, head-ache, fatigue and drowsiness. High concentrations can produce central nervous system depression and optic nerve damage. 50,000 ppm will probably cause death in 1 to 2 hrs. Can be absorbed through skin. Swallowing may cause death or eye damage. | [Fire Hazard]
Behavior in Fire: Containers may explode. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Shipping]
UN1230 Methanol, Hazard Class: 3; Labels:
3-Flammable liquid, 6.1-Poisonous material. (International) | [Incompatibilities]
Methanol reacts violently with strong
oxidizers, causing a fire and explosion hazard. | [Description]
Methyl alcohol, also known as methanol or wood alcohol, is a clear, colorless, flammable liquid
that is the simplest alcohol.
World production of methanol is approximately 8.5 billion gallons annually. Methanol
is produced industrially, starting with the production of synthesis gas or syngas. Syngas used
in the production of methyl alcohol is a mixture of carbon monoxide and hydrogen formed
when natural gas reacts with steam or oxygen. Methyl alcohol is then synthesized
from carbon monoxide and hydrogen.
Methyl alcohol is poisonous and is commonly used to denature ethyl alcohol. Methanol
poisoning results from ingestion, inhalation of methanol vapors, or absorption through
the skin. Methanol is transformed in the body to formaldehyde (H2CO) by the enzyme
alcohol dehydrogenase.The formaldehyde is then metabolized to formic acid (HCOOH)by aldehyde dehydrogenase. | [Waste Disposal]
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration | [Physical properties]
Clear, colorless liquid with a characteristic alcoholic odor. Odor threshold concentrations ranged
from 8.5 ppbv (Nagata and Takeuchi, 1990) to 100.0 ppmv (Leonardos et al., 1969).
Experimentally determined detection and recognition odor threshold concentrations were 5.5
mg/m3 (4.2 ppmv) and 69 mg/m3 (53 ppmv), respectively (Hellman and Small, 1974). | [History]
It was first isolated in 1661 by the Irish chemist Robert Boyle
(1627–1691) who prepared it by the destructive distillation of boxwood, giving it the name
spirit of box, and the name wood alcohol is still used for methyl alcohol. Methyl alcohol is also
called pyroxylic spirit; pyroxylic is a general term meaning distilled from wood and indicates
that methyl alcohol is formed during pyrolysis of wood. The common name was derived in the
mid-1800s. The name methyl denotes the single carbon alkane methane in which a hydrogen
atom has been removed to give the methyl radical. The word alcohol is derived from Arabic
al kuhul. | [Definition]
ChEBI: The primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. | [Production Methods]
Modern industrial-scale methanol production is exclusively
based on synthesis from pressurized mixtures of hydrogen,
carbon monoxide, and carbon dioxide gases in the presence
of catalysts. Based on production volume, methanol has
become one of the largest commodity chemicals produced
in the world. | [Reactions]
Methyl alcohol is a versatile material, reacting (1) with sodium metal, forming sodium methylate, sodium methoxide CH3ONa plus hydrogen gas, (2) with phosphorus chloride, bromide, iodide, forming methyl chloride, bromide, iodide, respectively, (3) with H2SO4 concentrated, forming dimethyl ether (CH3)2O, (4) with organic acids, warmed in the presence of H2SO4, forming esters, e.g., methyl acetate CH3COOCH3, [CAS: 79-20-9], methyl salicylate C6H4(OH)·COOCH3, possessing characteristic odors, (5) with magnesium methyl iodide in anhydrous ether (Grignard’s solution), forming methane as in the case of primary alcohols, (6) with calcium chloride, forming a solid addition compound 4CH3OH·CaCl2, which is decomposed by H2O, (7) with oxygen, in the presence of heated smooth copper or silver forming formaldehyde. The density of pure methyl alcohol is 0.792 at 20 °C compared with H2O at 4 °C (the corresponding figure for ethyl alcohol is 0.789), and the percentage of methyl alcohol present in a methyl alcohol-water solution may be determined from the density of the sample. | [World Health Organization (WHO)]
Methanol has been subjected to abuse by consumption as a
substitute for ethanol. Its toxic metabolites cause irreversible blindness and severe
metabolic acidosis, and are ultimately fatal. Methanol continues to be used as an
industrial solvent. | [Flammability and Explosibility]
Methanol is a flammable liquid (NFPA rating = 3) that burns with an invisible flame
in daylight; its vapor can travel a considerable distance to an ignition source and
"flash back." Methanol-water mixtures will burn unless very dilute. Carbon dioxide
or dry chemical extinguishers should be used for methanol fires. | [reaction suitability]
reaction type: volumetric | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization:Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Potential Exposure]
Drug,Mutagen; Reproductive Effector; Human Data; PrimaryIrritant. Methyl alcohol is used as a starting material inorganic synthesis of chemicals, such as formaldehyde,methacrylates, methyl amines, methyl halides, ethylene glycol, and pesticides, and as an industrial solvent for inks, resins, adhesives, and dyes. It is an ingredient in paint and varnish removers, cleaning and dewaxing preparations, spirit duplicating fluids, embalming fluids, antifreeze mixtures, and enamels, and is used in the manufacture of photographic film, plastics, celluloid, textile soaps, wood stains, coated fabrics, shatterproof glass, paper coating, waterproofing formulations, artificial leather, and synthetic indigo and other dyes. It has also been used as an extractant in many other processes, an antidetonant fuel-injection fluid for aircraft, a rubber accelerator, and a denaturant for ethyl alcohol. | [Source]
Methanol occurs naturally in small-flowered oregano (5 to 45 ppm) (Baser et al., 1991),
Guveyoto shoots (700 ppb) (Baser et al., 1992), orange juice (0.8 to 80 ppm), onion bulbs,
pineapples, black currant, spearmint, apples, jimsonweed leaves, soybean plants, wild parsnip,
blackwood, soursop, cauliflower, caraway, petitgrain, bay leaves, tomatoes, parsley leaves, and
geraniums (Duke, 1992).
Methanol may enter the environment from methanol spills because it is used in formaldehyde
solutions to prevent polymerization (Worthing and Hance, 1991). | [Environmental Fate]
Biological. In a 5-d experiment, [14C]methanol applied to soil water suspensions under aerobic
and anaerobic conditions gave 14CO2 yields of 53.4 and 46.3%, respectively (Scheunert et al.,
1987). Heukelekian and Rand (1955) reported a 5-d BOD value of 0.85 g/g which is 56.7% of the
ThOD value of 1.50 g/g. Using the BOD technique to measure biodegradation, the mean 5-d BOD
value (mM BOD/mM methanol) and ThOD were 0.93 and 62.0%, respectively (Vaishnav et al.,
1987).
Photolytic. Photooxidation of methanol in an oxygen-rich atmosphere (20%) in the presence of
chlorine atoms yielded formaldehyde and hydroxyperoxyl radicals. The reaction is initiated via
hydrogen abstraction by OH radicals or chlorine atoms yielding a hydroxymethyl radical.
Chlorine, formaldehyde, carbon monoxide, hydrogen peroxide, and formic acid were detected
(Whitbeck, 1983). Reported rate constants for the reaction of methanol and OH radicals in the
atmosphere: 5.7 x 10-11 cm3/mol·sec at 300 K (Hendry and Kenley, 1979), 5.7 x 10-8 L/mol·sec
(second-order) at 292 K (Campbell et al., 1976), 1.00 x 10-12 cm3/molecule·sec at 292 K (Meier et
al., 1985), 7.6 x 10-13 cm3/molecule·sec at 298 K (Ravishankara and Davis, 1978), 6.61 x 10-13
cm3/molecule·sec at room temperature (Wallington et al., 1988a). Based on an atmospheric OH
concentration of 1.0 x 106 molecule/cm3, the reported half-life of methanol is 8.6 d (Grosjean,
1997).
Chemical/Physical. In a smog chamber, methanol reacted with nitrogen dioxide to give methyl
nitrite and nitric acid (Takagi et al., 1986). The formation of these products was facilitated when
this experiment was accompanied by UV light (Akimoto and Takagi, 1986).
Methanol will not hydrolyze because it does not have a hydrolyzable functional group (Kollig,
1993).
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 964 mg/L. The adsorbability of the carbon used was 7 mg/g carbon (Guisti et al.,
1974).
Hydroxyl radicals react with methanol in aqueous solution at a reaction rate of 1.60 x 10-12
cm3/molecule?sec (Wallington et al., 1988).
Complete combustion in air produces carbon dioxide and water. The stoichiometric equation for
this oxidation reaction is:
2CH4O + 3O2 → 2CO2 + 4H2O | [storage]
Methanol should
be used only in areas free of ignition sources, and quantities greater than 1 liter
should be stored in tightly sealed metal containers in areas separate from oxidizers. | [Purification Methods]
Almost all methanol is now obtained synthetically. Likely impurities are water, acetone, formaldehyde, ethanol, methyl formate and traces of dimethyl ether, methylal, methyl acetate, acetaldehyde, carbon dioxide and ammonia. Most of the water (down to about 0.01%) can be removed by fractional distillation. Drying with CaO is unnecessary and wasteful. Anhydrous methanol can be obtained from "absolute" material by passage through Linde type 4A molecular sieves, or by drying with CaH2, CaSO4, or with just a little more sodium than required to react with the water present, in all cases the methanol is then distilled. Two treatments with sodium reduces the water content to about 5 x 10-5%. [Friedman et al. J Am Chem Soc 83 4050 1961.] Lund and Bjerrum [Chem Ber 64 210 1931] warmed clean dry magnesium turnings (5g) and iodine (0.5g) with 50-75mL of "absolute" methanol in a flask until the iodine disappeared and all the magnesium was converted to the methoxide. Up to 1L of methanol was added and, after refluxing for 2-3hours, it was distilled off, excluding moisture from the system. Redistillation from tribromobenzoic acid removes basic impurities and traces of magnesium oxides, and leaves conductivity-quality material. The method of Hartley and Raikes [J Chem Soc 127 524 1925] gives a slightly better product. This consists of an initial fractional distillation, followed by distillation from aluminium methoxide, and then ammonia and other volatile impurities are removed by refluxing for 6hours with freshly dehydrated CuSO4 (2g/L) while dry air is passed through: the methanol is finally distilled. (The aluminium methoxide is prepared by warming with aluminium amalgam (3g/L) until all the aluminium has reacted. The amalgam is obtained by warming pieces of sheet aluminium with a solution of HgCl2 in dry methanol.) This treatment also removes aldehydes. If acetone is present in the methanol, it is usually removed prior to drying. Bates, Mullaly and Hartley [J Chem Soc 401 1923] dissolved 25g of iodine in 1L of methanol and then poured the solution, with constant stirring, into 500mL of M NaOH. Addition of 150mL of water precipitated iodoform. The solution was allowed to stand overnight, filtered, then boiled under reflux until the odour of iodoform disappeared, and fractionally distilled. (This treatment also removes formaldehyde.) Morton and Mark [Ind Eng Chem (Anal Edn) 6 151 1934] refluxed methanol (1L) with furfural (50mL) and 10% NaOH solution (120mL) for 6-12hours, the refluxing resin carries down with it the acetone and other carbonyl-containing impurities. The alcohol was then fractionally distilled. Evers and Knox [J Am Chem Soc 73 1739 1951], after refluxing 4.5L of methanol for 24hours with 50g of magnesium, distilled off 4L of it, which they then refluxed with AgNO3 for 24hours in the absence of moisture or CO2. The methanol was again distilled, shaken for 24hours with activated alumina before being filtered through a glass sinter and distilled under nitrogen in an all-glass still. Material suitable for conductivity work was obtained. Variations of the above methods have also been used. For example, a sodium hydroxide solution containing iodine has been added to methanol and, after standing for 1day, the solution has been poured slowly into about a quarter of its volume of 10% AgNO3, shaken for several hours, then distilled. Sulfanilic acid has been used instead of tribromobenzoic acid in Lund and Bjerrum's method. A solution of 15g of magnesium in 500mL of methanol has been heated under reflux, under nitrogen, with hydroquinone (30g), before degassing and distilling the methanol, which was subsequently stored with magnesium (2g) and hydroquinone (4g per 100mL). Refluxing for about 12hours removes the bulk of the formaldehyde from methanol: further purification has been obtained by subsequent distillation, refluxing for 12hours with dinitrophenylhydrazine (5g) and H2SO4 (2g/L), and again fractionally distilling. [Beilstein 1 IV 1227.] | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) is 20,000μg/m3 with 4-hour averaging time. |
| Questions And Answer | Back Directory | [Introduction]
Methanol is the simplest fatty alcohol. It is a colorless, flammable, irritating liquid with a boiling point of 64.7°C, a melting point of -93.90°C, and a relative density of 0.7913. Soluble in water and most organic solvents. Its severe toxicity can damage the optic nerve. Once swallowed, it can make the eyes blind and even cause death.
Methanol has the general properties of a primary aliphatic alcohol. The three hydrogen atoms on a carbon atom with a hydroxyl group can be oxidized, orderly generating formaldehyde, formic acid, and carbon dioxide. Therefore, it is largely used in the synthesis of formaldehyde. Methanol is easily converted into important organic synthesis intermediates such as methyl carboxylate, methyl chloride and methylamine, and it is also applied as important organic solvents, extraction agents and alcohol denaturants.
| [Chemical Properties]
Methanol is a clear, colorless liquid with a characteristic pungent odor (NTP NIEHS web accessed 2/16/2013). The air odor threshold has been reported as 1500 ppm (approximately 2000mg/m3), much higher than the occupational guidelines.

Methanol (methyl alcohol; wood alcohol) is used extensively as a solvent for lacquers, paints, varnishes, cements, inks, dyes, plastics, and various industrial coatings. Large quantities are used in the production of formaldehyde and other chemical derivatives such as acetic acid, methyl halides and terephthalate, methyl methacrylate, and methylamines. Methanol is also used as a gasoline additive, as a component of lacquer thinners, in antifreeze preparations of the “nonpermanent” type, and in canned heating preparations of jellied alcohol. It is also used in duplicating fluid, in paint removers, and as a cleaning agent. The potential for methanol as a future alternative for gasoline indicates that the use of this chemical will most likely increase rather than decrease. At room temperature, methanol is a colorless liquid with a pungent odor. It is relatively volatile, with a vapor pressure of 96 mmHg and a vapor density of 1.11. It is miscible with water and soluble with other organic solvents. It is found in nature as a fermentation product of wood and as a constituent of some fruits and vegetables.
| [Uses]
Methanol is an important chemical raw material for fine chemicals. Its carbonylation at 3.5 MPa and 180-200° C in the presence of catalyst can produce acetic acid and further produce acetic anhydride. It reacts with syngas to prepare vinyl acetate in the presence of catalyst; reacts with isobutylene to produce tert-butyl methyl ether; prepare dimethyl oxalate through oxidization and carbonylation, and a further hydrogenation to produce ethylene glycol; reacts with toluene under catalyst and simultaneous oxidization to produce phenylethyl alcohol. It can be used as a good solvent, as a pesticide raw material, as an antifreeze agent, as a fuel and fuel additive (this is receiving increasing attention in environmental protection field). It is the main raw material in the preparation of formaldehyde, the raw material in medicine and spices production, a solvent in dyes and paint industries, the raw material in preparation of methanol single cell protein and synthesis of methyl ester.
Industry
Applications
Role/Benefit
Laboratory
HPLC, UV/VIS spectroscopy, and LCMS
Low UV cutoff
Chemical manufacture
Production of formaldehyde and its derivates
Main feedstock
Production of hydrocarbon chains and even aromatic systems
Main feedstock
Production of methyl tert-butyl ether
Methylation reagent
Production of dimethyl terephthalic acid, methyl methacrylate and acrylic acid methyl ester
Main feedstock
Plastics
Production of polymers
Main feedstock
Farm chemical
Production of insecticide and acaricide
Main feedstock
Pharmaceuticals
Production of sulfonamides, amycin, etc
Main feedstock
Fuel for vehicles
Pure methanol fuel
Pure methanol does not produce an opaque cloud of smoke in the event of an accident
Methanol gasoline
Blended directly into gasoline to produce a high-octane, efficient fuel with lower emissions than conventional gasoline
Chemical analysis
Determination of boron
Analysis agent
Determination of trace moisture in alcohols, saturated hydrocarbons, benzene, chloroform, pyridine
Analysis agent
Others
Separation of calcium sulfate and magnesium sulfate
Separation reagent
Separation of strontium bromide and barium bromide
Separation reagent
Anti-freezing agent
Effective component
| [Methanol poisoning and First Aid Measures]
Pathogenesis
First, methanol has a cumulative effect and is oxidized in the body into more toxic formaldehyde and formic acid. Methanol and its oxides directly damage the tissues, causing cerebral edema, meningeal hemorrhage, optic nerve and retinal atrophy, pulmonary congestion and edema, and hepatic and renal turbid swelling.
Second, methanol and its oxides cause blood circulation disorder, coenzyme system obstacles in vivo, resulting in lack of oxygen supply to the brain cortical cells, metabolic disorders, and related neurological and psychiatric symptoms.
Third, methanol oxidation products combine with the iron in the cytochrome oxidase, which inhibits the intracellular oxidation process thus causing metabolic disorders, acidosis along with organic acid accumulation in the body, and nerve cells impair.
Treatment
- Keep away from the methanol dispersion area, excrete methanol from the body.
Antidote: Ethanol is an antidote to methanol poisoning. Ethanol can prevent methanol’s oxidation and promote its emission. Prepare 5% ethanol solution using 10% glucose solution, and drip slowly intravenously.
- Maintain electrolyte balance: maintain respiratory and circulatory function, provide with a large number of Vitamin B.
Treatment of acidosis: Administrate timely sodium bicarbonate solution or sodium lactate solution based on blood gas analysis, carbon dioxide binding force measurement and clinical performance.
- Prevent cerebral edemas actively, reduce intracranial pressure, improve fundus blood circulation, and prevent optic neuropathy if needed.
- Inject intravenously cytochrome C, polar fluid to restore cytochrome oxidase function.
- Control mental state by applying diazepam, perphenazine and the like.
Symptoms and treatments:
Acute pain
morphine, pethidine
Convulsions
phenobarbital, amimystrine, diazepam
Coma
caffeine sodium benzoate
Respiratory failure
nikethamide, theophylline
| [Reactivities of Methanol]
Methanol is the simplest aliphatic alcohol. It contains only one carbon atom. Unlike higher alcohols, it cannot form an olefin through dehydration. However, it can undergo other typical reactions of aliphatic alcohols involving cleavage of a C-H bond or O-H bond and displacement of the -OH group. Table 1 summarizes the reactions of methanol, which are classified in terms of their mechanisms. Examples of the reactions and products are given.
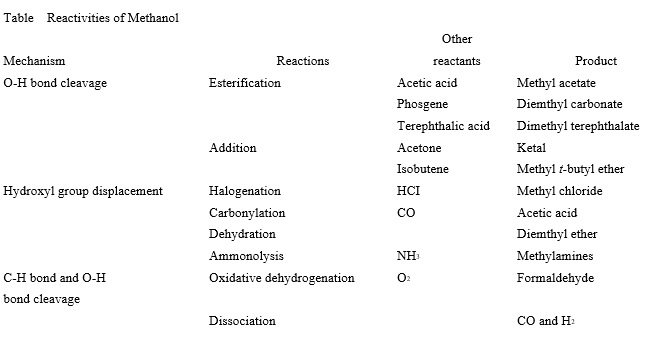
Homolytic dissociation energies of the C-O and O-H bonds in methanol are relatively high. Catalysts are often used to activate the bonds and to increase the selectivity to desired products. | [Production]
Methanol is prepared by pressure heating with carbon monoxide and hydrogen in the presence of a catalyst:
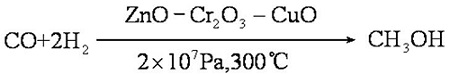
If the conditions are strictly controlled, the yield can reach 100% and the purity can reach 99%. Methane is mixed with oxygen (9:1, V/V) and methanol is obtained through a copper tube under heating and pressure:
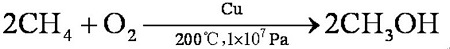 | [Toxicity evaluation]
ADI is limited to GMP (FAO/WHO, 2001).
Toxic, can cause blindness.
LD50: 5628 mg/kg (rat, oral).
Measurement
Date
System
Route/Organism
Dose
Effect
Skin and Eye Irritation
December 2016
eye /rabbit
40 mg
moderate
Skin and Eye Irritation
December 2016
eye /rabbit
100 mg/24H
moderate
Skin and Eye Irritation
December 2016
skin /rabbit
20 mg/24H
moderate
Mutation Data
December 2016
Cytogenetic Analysis
parenteral/grasshopper
3000 ppm
Mutation Data
December 2016
Cytogenetic Analysis
oral/mouse
1 gm/kg
Mutation Data
December 2016
Cytogenetic Analysis
intraperitoneal/mouse
75 mg/kg
Mutation Data
December 2016
DNA Damage
oral/rat
10 µmol/kg
Mutation Data
December 2016
DNA inhibition
lymphocyte/human
300 mmol/L
Mutation Data
December 2016
DNA repair
/Escherichia coli
20 mg/well
Mutation Data
December 2016
morphological transform
fibroblast/mouse
0.01 mg/L/21D (-enzymatic activation step)
from The National Institute for Occupational Safety and Health - NIOSH
| [Methanol gasoline]
Methanol gasoline refers to the M series mixture fuel made of addition of methanol to the gasoline and formulated using methanol fuel solvent. Among them, M15 (add 15% methanol in gasoline) clean methanol gasoline is used as vehicle fuel, respectively, used in a variety of gasoline engines. It can be applied to substitute the finished gasoline without changing the existing engine structure, and can also be mixed with refined oil. The methanol mixed fuel has excellent thermal efficiency, power, start-up and being economical. It is also characterized by lowering the emissions, saving oil and being safe and convenient. Methanol gasoline types of M35, M15, M20, M50, N85 and M100 with different blend ratios have been developed around the world according to the conditions of different countries. At present, the commercial methanol is mainly M85 (85% methanol + 15% gasoline) and M100 with M100 performance being better than M85 and having greater environmental advantages.
|
|
|