| Identification | More | [Name]
Chloromethyl pivalate | [CAS]
18997-19-8 | [Synonyms]
2,2-DIMETHYLPROPIONIC ACID CHLOROMETHYL ESTER
CHLOROMETHYL 2,2-DIMETHYLPROPIONATE
CHLOROMETHYL PIVALATE
CHLOROMETHYL PIVARATE
CHLOROMETHYL TRIMETHYLACETATE
CMPV
PIVALIC ACID CHLOROMETHYL ESTER
PIVALOYLOXYMETHYL CHLORIDE
POM
POM-CL
TRIMETHYLACETIC ACID CHLOROMETHYL ESTER
2,2-dimethyl-propanoicacichloromethylester
Pivaloxymethylchlorde
Chloromethyl pivalate, 99+%
Propanoic acid, 2,2-dimethyl-, chloromethyl ester
CHLORO METHYL PIVILATE
2,2-Dimethylpropanoic acid chloromethyl ester
Chloromethyl Pivalate [Amino-Protecting Agent]
Pivaloyloxymethyl chloride, POM-Cl
(2,2-Dimethyl-1-oxopropoxy)methyl chloride | [EINECS(EC#)]
242-735-1 | [Molecular Formula]
C6H11ClO2 | [MDL Number]
MFCD00000884 | [Molecular Weight]
150.6 | [MOL File]
18997-19-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R10:Flammable.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S41:In case of fire and/or explosion do not breathe fumes .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 3272 3/PG 3
| [WGK Germany ]
3
| [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29159000 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear colorless to slightly yellow liquid | [Uses]
Chloromethyl pivalate was used in the synthesis of pivaloyloxy methyl ester of ofloxacin as prodrug. It was used as the reagent during the synthesis of an isoindoline-annulated, tricyclic sultam library via microwave-assisted, continuous-flow organic synthesis. | [Application]
Chloromethyl pivalate is a pharmaceutical intermediate compound used in the synthesis of active pharmaceutical ingredients. It is also involved in the acylation reaction with 9-(2-phosphonomethoxyethyl)adenine. It is used as a protecting agent for the N-protection of amines. It is also used in the preparation of thivaloyloxymethyl ester of ofloxacin as a prodrug. It is also used in the preparation of sulbactam pivoxil by reaction with the sodium salt of sulbactam. Besides, it is used as a reagent in the synthesis of isoindoline cyclic and continuous flow organic synthesis. | [General Description]
Chloromethyl pivalate reacts with sodium salt of sulbactam to yield sulbactam pivoxil. It undergoes acylation reaction with 9-(2-phosphonylmethoxyethyl)adenine (PMEA) to yield bis(pivaloyloxymethyl) PMEA. | [Synthesis]
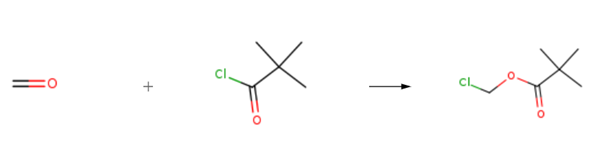
Chloromethyl pivalate is prepared by the reaction of formaldehyd and pivaloyl chloride. The specific synthesis steps are as follows:
A mixture of pivaloyl chloride (8.56 g, 71 mmol), paraformaldehyde (2.13 g, 71 mmol) and zinc chloride (75 mg, 0.55 mmol) was stirred at 80 °C for 2 h. Purification by vacuum distillation afforded chloromethyl pivalate (41) as a colourless oil (6.29 g, 44.7 mmol, 59%). bp 80 °C/15 mmHg [lit.1 bp 80-81 °C/15 mmHg]; 1H NMR (400 MHz, CDCl3) δ 1.24 (9H, s, tBu), 5.72 (2H, s, CH2).
 |
|
|