| Identification | More | [Name]
3-Morpholinopropanesulfonic acid | [CAS]
1132-61-2 | [Synonyms]
3-(4-MORPHOLINO)PROPANESULFONIC ACID
3-MORPHOLINOPROPANESULFONIC ACID
3-MORPHOLINOPROPANESULPHONIC ACID
3-(N-MORPHOLINO)PROPANESULFONIC ACID
3-(N-MORPHOLINO)PROPANESULPHONIC ACID
4-MORPHOLINEPROPANESULFONIC ACID
4-(MORPHOLINOPROPANE SULFONIC ACID)
LABOTEST-BB LT00455082
LABOTEST-BB LT01595956
MOPS
MOPS, CONCENTRATE SOLUTION
MORPHOLINOPROPANE SULFONIC ACID
MPS
TIMTEC-BB SBB009133
3-(4-Morpholino)propanesulphonic acid
3-N-morpholinopropansulfonic acid
MOPS 3-(N-Morpholino)propanesulfonic acid
MOPS, 10X Liquid Concentrate,Molecular Biology Grade
MOPS, Free Acid, MB Grade (1.01545)
MOPS, Free Acid, ULTROL Grade | [EINECS(EC#)]
214-478-5 | [Molecular Formula]
C7H15NO4S | [MDL Number]
MFCD00006183 | [Molecular Weight]
209.26 | [MOL File]
1132-61-2.mol |
| Chemical Properties | Back Directory | [Appearance]
White/clear crystalline powder | [Melting point ]
277-282 °C
| [bulk density]
350kg/m3 | [density ]
1.3168 (rough estimate) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.6370 (estimate) | [Fp ]
116 °C
| [storage temp. ]
Store at RT. | [solubility ]
H2O: 1 M at 20 °C, clear
| [form ]
Powder/Solid | [color ]
White | [Odor]
Odorless | [PH]
2.5-4.0 (25℃, 1M in H2O) | [PH Range]
6.5 - 7.9 | [pka]
7.2(at 25℃) | [Stability:]
Stable. Incompatible with strong bases, strong oxidizing agents. | [biological source]
synthetic | [Water Solubility ]
1000 g/L (20 ºC) | [λmax]
λ: 260 nm Amax: 0.020
λ: 280 nm Amax: 0.015 | [Detection Methods]
HPLC,NMR | [Merck ]
14,6265 | [BRN ]
1106776 | [InChIKey]
DVLFYONBTKHTER-UHFFFAOYSA-N | [LogP]
-2.94 at 20℃ | [CAS DataBase Reference]
1132-61-2(CAS DataBase Reference) | [EPA Substance Registry System]
1132-61-2(EPA Substance) | [Absorption]
≤0.05 at 260nm
≤0.05 at 280nm |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
1
| [RTECS ]
QE9104530
| [TSCA ]
Yes | [HS Code ]
29349990 |
| Questions And Answer | Back Directory | [Description]
MOPS (3-morpholinopropanesulfonic acid) is a buffer introduced by Good et al. in the 1960s. It is a structural analog to MES. Its chemical structure contains a morpholine ring. HEPES is a similar pH buffering compound that contains a piperazine ring. With a pKa of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH.It is used as a synthetic buffering agent below pH 7.5. | [application]
MOPS is frequently used as a buffering agent in biology and biochemistry. It has been tested and recommended for polyacrylamide gel electrophoresis. Usage above 20 mM in mammalian cell culture work is not recommended. MOPS buffer solutions become discolored (yellow) over time, but reportedly slight discoloration does not significantly affect the buffering characteristics. | [Reference]
P. H. Quail, D. Marme, E. Schäfer, Particle-bound phytochrome from maize and pumpkin, Nature New Biology, 1973, vol. 245, pp. 189-191
|
| Hazard Information | Back Directory | [Chemical Properties]
White/clear crystalline powder | [Uses]
Biological buffer. | [Definition]
ChEBI: 3-(N-morpholino)propanesulfonic acid is a Good's buffer substance, pKa = 7.2 at 20 ℃. It is a member of morpholines, a MOPS and an organosulfonic acid. It is a conjugate acid of a 3-(N-morpholino)propanesulfonate. It is a tautomer of a 3-(N-morpholiniumyl)propanesulfonate. | [General Description]
3-(N-Morpholino)propane sulfonic acid (MOPS) is an N-substituted amino sulfonic acid with a morpholinic ring. MOPS is capable of buffering within a pH range of 6.5-7.9. MOPS is widely used in biological and biochemical studies due to its inert properties. It does not interact with any metal ions in solutions and has significant metal-buffer stability especially with copper (Cu), nickel (Ni), manganese (Mn), zinc (Zn), cobalt (Co) ions. MOPS buffer maintains the pH of the mammalian cell culture medium. MOPS functions to maintain pH in denaturing gel electrophoresis of RNA. MOPS can modify lipid interactions and influence the thickness and barrier properties of membranes. MOPS interacts with bovine serum albumin and stabilizes the protein. Hydrogen peroxide oxidizes MOPS slowly to N-oxide form. | [Flammability and Explosibility]
Notclassified | [Synthesis]
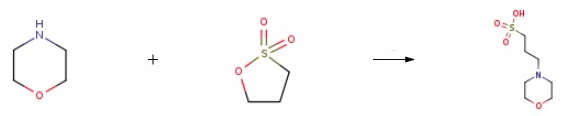
Morpholine and 1,3-Propanesultone are added to absolute ethanol for reaction. The crude product synthesized is purified to obtain 3-Morpholinopropanesulfonic acid.
 | [storage]
Store at -20°C |
|
|