| Identification | More | [Name]
1-Oxa-4-azacyclohexane | [CAS]
110-91-8 | [Synonyms]
1-OXA-4-AZACYCLOHEXANE
AKOS BBS-00003660
DIETHYLENEIMIDE OXIDE
DIETHYLENE IMIDOXIDE
DIETHYLENE OXIMIDE
LABOTEST-BB LTBB000400
MORPHOLIN
MORPHOLINE ON RASTA RESIN
TETRAHYDRO-1,4-OXAZINE
TETRAHYDRO-2H-1,4-OXAZINE
TETRAHYDRO-P-OXAZINE
1,4-Oxazinan
1,4-oxazine,tetrahydro-
2H-1,4-Oxazine, tetrahydro-
4H-1,4-Oxazine, tetrahydro-
BASF 238
basf238
Diethylenimide oxide
diethylenimideoxide
Drewamine | [EINECS(EC#)]
203-815-1 | [Molecular Formula]
C4H9NO | [MDL Number]
MFCD00005972 | [Molecular Weight]
87.12 | [MOL File]
110-91-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Morpholine is a colorless liquid with
a weak ammonia or fish-like odor. The odor threshold
is 0.01 ppm. | [Melting point ]
-7--5 °C (lit.) | [Boiling point ]
126.0-130.0 °C
129 °C (lit.) | [density ]
1.000 g/mL at 20 °C
| [vapor density ]
3 (vs air)
| [vapor pressure ]
31 mm Hg ( 38 °C)
| [refractive index ]
n20/D 1.454(lit.)
| [Fp ]
96 °F
| [storage temp. ]
Store at R.T. | [solubility ]
water: miscible | [form ]
Liquid | [pka]
8.33(at 25℃) | [color ]
APHA: ≤15 | [Specific Gravity]
0.996 | [Odor]
Characteristic amine-like odor | [PH]
11.2 (H2O)(undiluted) | [Stability:]
Stable. Flammable. Incompatible with strong oxidizing agents, strong acids, acid chlorides, acid anhydrides. Hygroscopic. | [explosive limit]
1.4-15.2%(V) | [Water Solubility ]
MISCIBLE | [FreezingPoint ]
-4.9℃ | [Sensitive ]
Hygroscopic | [Detection Methods]
GC | [Merck ]
14,6277 | [BRN ]
102549 | [Dielectric constant]
7.3(25℃) | [Exposure limits]
TLV-TWA 20 ppm (~70 mg/m3) (ACGIH,
MSHA, and OSHA); STEL skin 30 ppm
(ACGIH); IDLH 8000 ppm. | [InChIKey]
YNAVUWVOSKDBBP-UHFFFAOYSA-N | [LogP]
-0.860 | [Uses]
Rubber accelerator, solvent, additive to boilerwater, waxes and polishes, optical brightener fordetergents, corrosion inhibitor, preservation of bookpaper, organic intermediate (catalyst, antioxidants,pharmaceuticals, bactericides, etc.). | [CAS DataBase Reference]
110-91-8(CAS DataBase Reference) | [IARC]
3 (Vol. 47, 71) 1999 | [NIST Chemistry Reference]
Morpholine(110-91-8) | [EPA Substance Registry System]
110-91-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R10:Flammable.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R34:Causes burns. | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S36:Wear suitable protective clothing .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [OEB]
A | [OEL]
TWA: 20 ppm (70 mg/m3), STEL: 30 ppm (105 mg/m3) [skin] | [RIDADR ]
UN 2054 8/PG 1
| [WGK Germany ]
3
| [RTECS ]
QD6475000
| [Autoignition Temperature]
590 °F | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
I | [HS Code ]
29349900 | [Safety Profile]
Moderately toxic by
ingestion, inhalation, skin contact, and
intraperitoneal routes. Mutation data
reported. A corrosive irritant to skin, eyes,
and mucous membranes. Can cause kidney damage. Questionable carcinogen with
experimental neoplastigenic data.
Flammable liquid. A very dangerous fire
hazard when exposed to flame, heat, or
oxidizers; can react with oxidizing materials.
To fight fire, use alcohol foam, CO2, dry
chemical. Mixtures with nitromethane are
explosive. May ignite spontaneously in
contact with cellulose nitrate of high surface
area. When heated to decomposition it emits
highly toxic fumes of NOx. | [Hazardous Substances Data]
110-91-8(Hazardous Substances Data) | [Toxicity]
LD50 orally in female rats: 1.05 g/kg (Smyth) | [IDLA]
1,400 ppm [10% LEL] |
| Hazard Information | Back Directory | [General Description]
Morpholine(110-91-8) is a colorless to yellow liquid with a weak ammonia or fish-like odor. The odor threshold is 0.01 ppm. The reactivity of morpholine is mainly due to its secondary amine group. It readily undergoes organic condensations, alkylations, and arylations, resulting in the formation of various N-substituted morpholine compounds. Ethers are relatively chemically inert, hence the oxygen is of relatively little consequence except as a member of the heterocyclic ring (Texaco Chemical Co. 1982). | [Reactivity Profile]
MORPHOLINE dissolved in water neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. | [Air & Water Reactions]
Highly flammable. Water soluble. | [Hazard]
Flammable, moderate fire risk. Toxic byingestion and inhalation, irritant to skin, absorbedby skin. Eye damage and upper respiratory tractirritant. Questionable carcinogen. | [Potential Exposure]
Morpholine is used as a separating
agent for volatile amines; an intermediate for textile
lubricants; in the synthesis of rubber accelerators and pharmaceuticals.
It is also used as a solvent; as a boiler water
additive; and in the formulation of waxes, polishers and
cleaners. | [Fire Hazard]
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
Medical observation is recommended for 24 to 48 hours
after breathing overexposure, as pulmonary edema may be
delayed. As first aid for pulmonary edema, a doctor or
authorized paramedic may consider administering a drug or
other inhalation therapy. | [Shipping]
UN2054 Morpholine, Hazard class: 8; Labels:
8-Corrosive material, 3-Flammable liquid. | [Incompatibilities]
Strong acids, strong oxidizers; metals,
nitro compounds. Corrosive to metals; attacks copper and
its compounds. | [Description]
Morpholine is a colorless liquid with a weakammonia or fish-like odor. The odor threshold is 0.01 ppm.Molecular weight = 87.14; Specific gravity (H2O:1) 51.007; Boiling point = 128.9℃; Freezing/Meltingpoint 5 25℃; Vapor pressure 5 6 mmHg at 20℃; Flashpoint 5 37℃. Autoignition temperature 5 310℃. Explosivelimits: LEL 5 1.4%; UEL 5 11.2%. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 3, Reactivity 0. Soluble in water | [Waste Disposal]
Controlled incineration
(incinerator equipped with a scrubber or thermal unit to
reduce nitrogen oxides emissions). | [Physical properties]
Colorless, mobile, oily, hygroscopic, flammable liquid with a weak ammonia-like odor.
Experimentally determined detection and recognition odor threshold concentrations were 40 μg/m3
(11 ppbv) and 25 μg/m3 (70 ppbv), respectively (Hellman and Small, 1974). Forms explosive
vapors at temperatures >35 °C. | [Definition]
ChEBI: Morpholine is an organic heteromonocyclic compound whose six-membered ring contains four carbon atoms and one nitrogen atom and one oxygen atom that lies opposite to each other; the parent compound of the morpholine family. It is a saturated organic heteromonocyclic parent and a member of morpholines. It is a conjugate base of a morpholinium. | [Production Methods]
Morpholine is produced by reacting diethylene glycol, ammonia, and a small amount of hydrogen over a hydrogenation catalyst at 150-400°C and 30-400 atmospheres with the morpholine being recovered by fractional distillation. Various byproducts include 2-(2-aminoethoxy)ethanol and Af-alkylmorpholines (NRC 1981). | [Health Hazard]
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. Morpholine is readily absorbed through the skin; it causes nasal irritation when inhaled, with coughing, bronchial irritation, and pulmonary edema at increasingly higher concentrations. Upon ingestion, it causes hemorrhage in the gastrointestinal tract, with possible diarrhea; liver and kidney damage may occur if sufficient amounts are ingested or inhaled. Morpholine itself is not a carcinogen on the basis of available data. | [Flammability and Explosibility]
Flammable(100%) | [Industrial uses]
The total industrial consumption of morpholine is 11,000 metric tons/year. The largest usage for morpholine (33%) is in the rubber industry as an intermediate in the production of delayed-action accelerators for the polymerization of rubber, as stabilizers against heat-aging effects, and as bloom inhibitors in butyl rubber vulcanization. A second large proportion (25%) of morpholine production is used as an inhibitor to combat carbonic acid corrosion in condensate return lines of steam boiler systems. Morpholine is an intermediate in the manufacture of optical brighteners utilized by the soap and detergent industry. Morpholine reacts readily with fatty acids, forming soaps used in the formulation of self-polishing waxes and polishes and in coatings for the food industry. N-methyl morpholine and TV-ethyl morpholine are used as catalysts in the manufacture of polyurethane foams. Morpholine derivatives are utilized in pharmaceutical applications, as bactericides, fungicides, and herbicides, and as separating agents for oils. Other derivatives are utilized in the textile and printing industry as adjuvants, whitening agents, stabilizers, ink eradicators, and paper conditioners (Mjos 1978; NRC 1981; Texaco Chemical Co. 1982). | [Toxicology]
Common signs of toxicity following repeated dosing are local irritation and inflammations of the stomach, respiratory tract, and eyes, as well as systemic effects primarily on the liver and kidneys. In rats, exposure to 250 mL/m3 (890 mg kg-1 d-1, 6 h/d, 5 d/week, 90 d) and to up to 150 mL/m3 (543 mg/m3, 6 h/d, 5 d/week, 104 weeks) produced focal erosions and squamous-cell metaplasia of the nasal cavities and turbinates and ocular irritation, but no hematological or organ effects; at 90 mg/m3 (25 mL/m3, subchronic) and 36 mg/m3 (10 mL/m3, chronic), no treatment-related effects at all were identified. These data may be taken as NOAELs, although one earlier Russian publication claimed that some adverse effects were conspicuous on the spleen and in the red and white blood counts in rats and guinea pigs after four-month inhalation of 70 mg/m3 and less.
| [Carcinogenicity]
Morpholine did not produce an
increase in tumors in rats that inhaled from 10 to 150 ppm for
2 years. No tumors were seen in rats fed 5000 ppm
morpholine for 8 weeks and observed for their lifetime.
Morpholine fed concurrently with sodium nitrate increased
the numbers of hepatocellular carcinomas and sarcomas
of the liver and lungs of rats and mice, probably mediated
through the formation of N-nitrosomorpholine.
The authors concluded that morpholine itself was either
weakly carcinogenic or that a nitrate from an unknown source
was present. No cancers were produced when 6330 ppm
morpholine was added to the drinking water of mice for
their lifetimes. Concurrent exposure of morpholine
plus nitrite or nitrogen dioxide increased the tumor incidence
in a variety of species. In a feeding
study where morpholine (0.5% in diet) and sodium
nitrate were given concurrently for 23 weeks, rats showed no
evidence of cancer. | [Environmental Fate]
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 0.0 g/g which is 0.0% of
the ThOD value of 1.84 g/g.
Poupin et al. (1998) isolated a Mycobacterium strain RP1 from a contaminated activated sludge
that utilized morpholine as the sole source of carbon, nitrogen, and energy. The investigators
proposed the following degradation pathway: 2-hydroxymorpholine → (2-(2-aminoethoxy)acetaldehyde
→ 2-(2-aminoethoxy)acetate → glycolate and ethanolamine.
Chemical/Physical. In an aqueous solution, chloramine reacted with morpholine to form Nchloromorpholine
(Isaac and Morris, 1983). The aqueous reaction of nitrogen dioxide (1–99 ppm)
and morpholine yielded N-nitromorpholine (Cooney et al., 1987).
Slowly decomposes in the absence of oxygen. | [Metabolism]
Early reports indicated that morpholine was excreted unchanged after administration to rats (Tanaka et al 1978), dogs (Rhodes and Case 1977), and rabbits (Van Stee et al 1981). Sohn et al (1982b, 1982c) reported that approximately 80% of a radioactive dose was excreted in the urine within 24 h when administered intraperitoneally to rats, hamsters, and guinea pigs. Although 99% of the excreted dose was unmetabolized in the rat and hamster, 20% of the dose appeared in the urine of guinea pigs as N-methylmorpholine-N-oxide. N-Hydroxymorpholine and N-methylmorpholine were also detected in extracts of guinea pig tissues. Studies of the metabolism of morpholine-containing pharmaceutical agents in humans and animals indicate that the morpholine moiety may be hydroxylated or oxidized at C2 and C3, with subsequent ring cleavage (Oelschlager and Al Shaik 1985).
| [storage]
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist.Morpholine must be stored to avoid contact with strongacids (such as nitric acid) and strong oxidizers (such aschlorine, chlorine dioxide, bromine, nitrates, and permanganates) since violent reactions occur. | [Purification Methods]
Dry morpholine with KOH, fractionally distil it, then reflux it with Na, and again fractionally distil it. Dermer & Dermer [J Am Chem Soc 59 1148 1937] precipitated it as the oxalate by adding slowly to slightly more than 1 molar equivalent of oxalic acid in EtOH. The precipitate is filtered off and recrystallised twice from 60% EtOH [1:1 salt has m 190-195o(dec)]. Addition of the oxalate to concentrated aqueous NaOH regenerated the base, which is separated and dried with solid KOH, then sodium, before being fractionally distilled. The hydrochloride has m 178-179o (from MeOH/Et2O), and the picrate has m 151.6o (from aqueous EtOH). [Beilstein 27 II 3, 27 III/IV 15.] |
| Questions And Answer | Back Directory | [Product Features]
Morpholine, also known as 1, 4-oxazepine and diethylenimine oxide, is a kind of colorless alkaline oily liquid. It smells of ammonia and has hygroscopicity. Morpholine could evaporate with water vapor and miscible with water. It is Soluble in acetone, benzene, ether, pentane, methanol, ethanol, carbon tetrachloride, propylene glycol and other organic solvents. Morpholine Vapor could form an explosive mixture with air and the explosion limit is 1.8% to 15.2% (volume fraction). Morpholine is a secondary amine, and at the same time it has the property of inorganic acid and organic acid, so that it can generate salt and amide.
Morpholine contains secondary amine groups and it has all the typical reaction characteristics of the secondary amine groups. It can react with inorganic acid to form a salt, and also can react with organic acid to form salt or amide. Morpholine can carry out alkylation reactions and it also carry out ketone reaction or Willgerodt reaction with ethylene oxide. Because of the unique chemical properties of morpholine, it has become one of the important petrochemical products with important commercial application. It can be applied to produce rubber vulcanization accelerators such as NOBS, DTOS and MDS. And it is also applied to produce anti-corrosives, anti-corrosion agents, detergents, detergents, analgesics, local anesthetics, sedatives, respiratory and vascular stimulants, surfactants, optical bleach, fruit preservatives, and textile dyeing auxiliaries. Morpholine also has a wide range of applications in the field of rubber, pharmaceuticals, pesticides, dyes, coatings and other industries. In medication it could be applied in the production of morpholine guanidine, virus Ling, ibuprofen, cough must, naproxen, dichloroaniline, sodium phenylacetate and other important drugs.
The two main production method of morpholine are DEA method (diethanolamine method) and DEA method (diethylene glycol method)
It is noteworthy that new polymer monomer acrylic morpholine has obtained a rapid development in recent years. Acrylic acid morpholine could be produced from the reaction between acrylic acid and morpholine. And acrylic acid morpholine is a kind of water-soluble monomer, and it is still water-soluble after the polymerization. So it could be applied for the modification of aqueous polymers. Besides, acrylic morpholine is widely used as a reactive diluent for UV curable resins. With the deepening of applied research, many new specific uses have been developed and it becomes polymer monomer with rapid development.
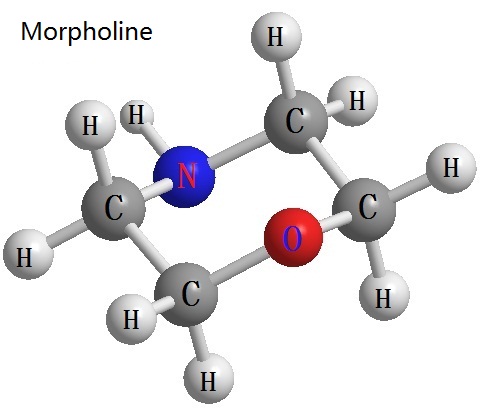
Figure 1 for the morpholine structure | [Chemical properties]
It is colorless water-absorbing oily liquid and it smells ammonia. It is soluble in water and methanol, ethanol, benzene, acetone, ether, ethylene glycol and other commonly used solvents. | [Uses]
(1) For medicine, it used as the raw materials for rubber accelerator and fluorescent whitening agent.
(2) Morpholine is the intermediates of fungicide dimethomorph and flumorpholine and organophosphate insecticide phosalphos.
(3) Morpholine is Mainly used for the production of rubber vulcanization accelerator, but also for surfactant, textile auxiliaries, pharmaceuticals, pesticide synthesis. (4) Morpholine also used as a catalyst for polymerization of butadiene, corrosion inhibitors, optical bleach, the goods are dyes, resins, wax, early glue, casein and other solvents. At present, the total production of morpholine in the world is 3-4 million t/a.
(5) Morpholine salts are also widely used. Morpholine salts like morpholine hydrochloride (10024-89-2) are the organic synthesis of intermediates. Morpholine fatty acid salt can be used as the coating agent of fruits or vegetables epidermal coating agent, and it could inhibit the base respiration and prevent the epidermis from evaporation of water and epidermal atrophy.
(6) Morpholine is the main raw materials of accelerator NOBS. for analysis reagents and resins, wax, shellac and other solvents, used in the production of sodium sulfate. Water glass and ultramarine. Also used in the manufacture of glass. Paper. Detergent. Soap. Dye. Synthetic fiber. Tanning. Medicine and ceramics industries. Analysis reagents, such as nitrogen determination, dehydrating agent.
(7) Morpholine is used for the analysis of reagents and resins, wax, casein, shellac and a variety of solvents solvent.
(8) Morpholine could produce salt after reacting with inorganic acid, and it also could produce salt or amide after reacting with organic acid. It also can be alkylated, and it also could come up a ketone reaction or Willgerodt reaction with ethylene oxide. | [metal corrosion inhibitors]
As a kind of metal corrosion inhibitor, Morpholine is mainly applied for the anti-corrosion of iron, copper, zinc, lead and other metals. It remains at its starting stage in China, but outside the country, a considerable proportion of Morpholine are used as a kind of anti-rust agent for metal gas to prevent the metal corrosion caused by atmosphere and it has been widely used in the field of mechanical instruments, automobiles, medical equipment and others. The metal atmospheric rust inhibitor used earlier such as dicyclohexylamine nitrite and cyclohexylamine do harm to the human body and they are more environmentally toxic. Instead, morpholine as a metal gas-liquid corrosion inhibitor, it has the advantages of low toxicity, so the foreground is prosperous. | [rubber vulcanization accelerator]
Before 1990's, in Europe, the United States, Japan and other developed areas, the consumption of rubber vulcanization accelerator accounted for more than 50% of the total demand for morpholine. At present, more than 30% consumption of rubber vulcanization accelerator is used for NOBS.
In recent years, the toxicity issue of the harmful nitrosamines in the accelerator which produced during rubber processing is driving more attention internationally. A number of restrictive laws and regulations are introduced all around the world. For example, in Germany as early as 1982, the law about the regulations issued nitrosamine content control was established. The United States, Japan, France, the United Kingdom actively developed new vulcanization accelerator which don’t produce nitrosamine, and they have stopped using nitrosamine which will produce vulcanization accelerator. Therefore, the consumption of morpholine in foreign countries has decreased with banning of the accelerator NOBS year by year. In China however, we have no corresponding regulations prohibited about banning accelerator NOBS. But China has joined the WTO, due to a large number of foreign enter and high requirement about additives localization, it requires more environmental protection in China's rubber vulcanization accelerator. Using non-toxic promoter and replacing NOBS becomes the general trend. China's demand for NOBS is significantly reduced in recent years. China will no longer use basic decomposition of nitrosamine secondary amine accelerator. As the main substitute of NOBS, NS (N-tert-butyl-2-benzothiazole sulfonamide), which does not produce nitrosamines, has a large production capacity. In 2005, the output was 14,000 tons and it is 10.1%, of the total amount. So there was good development momentum. | [quantitative analysis]
Get 4 parts of methanol solution with 0.1% of bromocresol green and 1 part of 0.1% aqueous solution with 0.1% of methyl red sodium salt. Mix them and put aside to use as a mixed indicator later.
Get 50ml water in 250ml flask, add 0.4ml mixed indicator, drop 0.1moI/L hydrochloric acid, and wait for the color to turn green. Accurately weighs the sample 1.4~1.6g, add the flask and mix it. Use 0.5mol/L hydrochloric acid titration and wait for the color to turn green. Per ml, 0.5mol/L hydrochloric acid is equivalent to 43.56mg C4H9NO. | [Production method]
(1) Morpholine could be produced by diethanolamine dehydration cyclization derived from sulfuric acid. Add diethanolamine to the water reaction pot and drop sulfuric acid in the temperature of 60 ℃, then when the temperature heat at 185-195 ℃, incubate it for 30min. Cool it to under 60℃ and drop sodium hydroxide solution to pH = 11. The next steeps are cooling, filtering, filtrating distillation, collecting the following fractions below 130℃. The content of spermine is suppose to reach more than 99.5%. The method is easy to obtain raw materials, so it has become the main method of producing morpholine in the world. Morpholine also could be produced in the catalytic reaction between dioxane and ammonia gas.
(2) The preparation method is that we could get Morpholine from the presence of sulfuric acid dehydration cyclization diethanolamine In the presence of sulfuric acid ; then add diethanolamine into the reaction kettle and add H2SO4 at a temperature of below 6 °C, then heat it to 185-195 °C for 30 minutes, cool to 60 °C. Drop NaOH solution to pH = 11, and the last two steeps is cooling and filtrating. Morpholine could be collected from the fraction below 130 °C.
We can also get morpholine from the reaction between diethylene glycol and ammonia in the presence of catalyst and pressure. The method is easy to obtain raw materials, thus it is the main method of producing morpholine around the world. | [Hazards & Safety Information]
category: Flammable liquids
toxicity grading: Poisoning
acute toxicity: Oral-rat LD 50: 1050 mg/kg; oral-mouse LD 50: 525 mg/kg
Stimulating data: Skin-rabbit 995 mg/24 h severe; eye –rabbit 2 mg severe.
hazardous characteristic of explosive: Will cause explosion when it mixes with steam and air
hazardous characteristic of flammability: Flammable; combustion produces toxic chloride gas
transportation and storing properties: Store it in low temperature, dry and ventilated environment. Prevent from fire, friction�,spark and Separated from oxidant
extinguishant: Water spray, dry chemical powder, foam or carbon dioxide
occupational standards: TLV-TWA 70 mg/m3 STEL 105 mg/m3 |
|
|