| Identification | More | [Name]
Taurine | [CAS]
107-35-7 | [Synonyms]
2-AMINOETHANE-1-SULFONIC ACID
2-AMINOETHANESULPHONIC ACID
2-AMINOETHYLSULFONIC ACID
2-AMINOETHYLSULPHONIC ACID
2-AMINOMETHANESULPHONIC ACID
AMINOETHYLSULFONIC ACID
AMINOETHYLSULPHONIC ACID
FEMA 3813
TAURINE
TURIN
2-amino-ethanesulfonicaci
2-sulfoethylamine
Aminoethanesulfonic acid
beta-Aminoethylsulfonic acid
Ethanesulfonic acid, 2-amino-
Ethanesulfonicacid,2-amino-
nci-c60606
O-Due
tauphon
Ethylaminosulfonic acid
| [EINECS(EC#)]
203-483-8 | [Molecular Formula]
C2H7NO3S | [MDL Number]
MFCD00008197 | [Molecular Weight]
125.15 | [MOL File]
107-35-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
>300 °C (lit.) | [density ]
1.00 g/mL at 20 °C
| [FEMA ]
3813 | [refractive index ]
1.5130 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
H2O: 0.5 M at 20 °C, clear, colorless
| [form ]
Crystals or Crystalline Powder | [pka]
1.5(at 25℃) | [color ]
White | [Odor]
bland | [PH]
4.5-6.0 (25℃, 0.5M in H2O) | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic | [Water Solubility ]
5-10 g/100 mL at 23.5 ºC | [λmax]
λ: 260 nm Amax: 0.006
λ: 280 nm Amax: 0.005 | [Detection Methods]
T,NMR | [JECFA Number]
1435 | [Merck ]
14,9074 | [BRN ]
1751215 | [InChIKey]
XOAAWQZATWQOTB-UHFFFAOYSA-N | [LogP]
-3.36--1.2 at 20℃ | [CAS DataBase Reference]
107-35-7(CAS DataBase Reference) | [NIST Chemistry Reference]
2-Aminoethanesulfonic acid(107-35-7) | [EPA Substance Registry System]
107-35-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
2
| [RTECS ]
WX0175000
| [TSCA ]
Yes | [HS Code ]
29211980 | [Safety Profile]
Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of SOx and NOx. | [Hazardous Substances Data]
107-35-7(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: > 5000 mg/kg |
| Hazard Information | Back Directory | [General Description]
Large white crystals or white powder. | [Reactivity Profile]
L-TAURINE(107-35-7) is an amino acid found in combination with bile acids [Hawley]. | [Air & Water Reactions]
Water soluble. | [Health Hazard]
ACUTE/CHRONIC HAZARDS: This material evolves highly toxic fumes when heated to decomposition, and may cause irritation on contact. | [Fire Hazard]
Flash point data are not available for this chemical, but L-TAURINE is probably combustible. | [Chemical Properties]
Taurine(107-35-7) is a naturally occurring amino acid with a bitter taste and sharp flavor. It is a nonessential amino acid by definition because the body can synthesize it from methionine and cysteine.
| [Chemical Properties]
White crystalline powder | [Occurrence]
Reported found in beef, black beans, chicken, chick peas, clams, cod, fish, lamb, milk, octopus, oysters, pistachios, pork, scallops, shrimp and other natural sources. | [Uses]
Taurine(107-35-7) is an organic acid found in animal tissues and is a major constituent of bile. Taurine has many biological roles such as conjugation of bile acids, antioxidation, osmoregulation, membrane stab
ilization and modulation of calcium signaling.
| [Uses]
vitamin B1, enzyme cofactor | [Definition]
ChEBI: An amino sulfonic acid that is the 2-amino derivative of ethanesulfonic acid. It is a naturally occurring amino acid derived from methionine and cysteine metabolism. An abundant component of fish- and meat-based foods, it has been used as an oral suppleme
t in the treatment of disorders such as cystic fibrosis and hypertension. | [Biosynthesis]
In addition to the intake of taurine directly from the diet, the animal body can also biosynthesis in the liver. The intermediate product of methionine and cysteine metabolism, cysteine, is decarboxylated to taurine by cysteine decarboxylase (CSAD), and then oxidized to taurine. CSAD is considered to be the rate limiting enzyme of taurine biosynthesis in mammals, and compared with other mammals, the activity of human CSAD is lower, which may be due to the low taurine synthesis ability in human body. Taurine can participate in the formation of taurocholic acid and hydroxyethyl sulfonic acid after decomposition in vivo. The amount of taurine required depends on cholic acid binding capacity and muscle content. | [Hazard]
Toxic by ingestion. | [Biological Activity]
One of the most abundant free amino acids in the brain. A partial agonist at the inhibitory glycine receptor. | [Biochem/physiol Actions]
Non-selective endogenous agonist at glycine receptors. Conditionally essential sulfonated amino acid which modulates apoptosis in some cells; functions in many metabolic activities; a product of methionine and cysteine metabolism. | [Pharmacology]
Taurine(107-35-7) is an organic osmotic regulator. It not only participates in the regulation of cell volume, but also provides the basis for the formation of bile salts. It also plays an important role in the modulation of intracellular free calcium concentration. Although taurine is a special amino acid not included in proteins, taurine is the most abundant amino acid in brain, retina and muscle tissue. Taurine is widely used, such as in the function of central nervous system, cell protection, cardiomyopathy, renal insufficiency, abnormal development of renal function and retinal nerve injury. Almost all eye tissues contain taurine. The quantitative analysis of rat eye tissue extract showed that taurine was the most abundant amino acid in retina, vitreous, lens, cornea, iris and ciliary body. Many studies have found that taurine is an active substance that regulates the normal physiological activities of the body. It has the functions of anti-inflammatory, analgesic, maintaining the osmotic pressure balance of the body, maintaining normal visual function, regulating the calcium balance of cells, reducing blood sugar, regulating nerve conduction, participating in endocrine activities, regulating lipid digestion and absorption, increasing the contractility of the heart, improving the immune capacity of the body, and enhancing the antioxidant capacity of cell membrane Protect a wide range of biological functions such as cardiomyocytes.
| [Veterinary Drugs and Treatments]
Taurine(107-35-7) has proven beneficial in preventing retinal degeneration
and the prevention and treatment of taurine-deficiency dilated
cardiomyopathy in cats. Although modern commercial feline diets
have added taurine, some cats still develop taurine-deficiency associated
dilated cardiomyopathy. It may also be of benefit in taurine
(±carnitine) deficient cardiomyopathy in American Cocker
Spaniels and certain other breeds such as, Golden Retrievers,
Labrador Retrievers, Newfoundlands, Dalmations, Portuguese
Water Dogs, and English Bulldogs. Preliminary studies have shown
evidence that it may be useful as adjunctive treatment for cardiac
disease in animals even if taurine deficiency is not present. Because
of its low toxicity, some have suggested it be tried for a multitude
of conditions in humans and animals; unfortunately, little scientific
evidence exists for these uses.
| [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Description]
Taruine(107-35-7) is an organic compounds that widely existing in animal tissues. It is a sulfur amino acid, but not being used for protein synthesis. It is rich in the brain, breasts, gallbladder and kidney. It is an essential amino acid in the pre-term and newborn infants of human. It has various kinds of physiological functions including being as a neurotransmitter in the brain, conjugation of bile acids, anti-oxidation, osmoregulation, membrane stabilization, modulation of calcium signaling, regulating the cardiovascular function as well as the development and function of skeletal muscle, the retina, and the central nervous system. It can be manufactured through the ammonolysis of isethionic acid or the reaction of aziridine with sulfurous acid. Because of its highly important physiological role, it can be supplied to energy drinks. It can also be used in cosmetics to maintain skin hydration, and used in some contact lens solution.
| [History]
As the conditionally essential amino acid of the human body, it is a kind of β- sulphamic acid. In mammalian tissues, it is a metabolite of methionine and cystine. It was first isolated from ox bile in 1827, hence the name taurine. It commonly exists in the form of free amino acids in various tissues of animals, but not goes into proteins without combination. Taurine is rarely found in plants. Early on, people had considered it a bile acid binding agent of taurocholic combined with cholic acid. However, recent studies have shown that taurine has many important biological functions apart from the above mentioned forming taurocholic acid and participating in the digestion and absorption of lipids.It is important nutrients for normal development and function of cranial nerve to play the role in adjusting a variety of nerve cells of the central nervous system; taurine in retina accounts for 40% to 50% of total free amino acid, which is necessary for maintaining the structure and function of photoreceptor cells; affecting the myocardial contracts dint, regulating calcium metabolism, controlling arrhythmia, lowering blood pressure, etc; maintaining cellular antioxidant activity to protect the tissues from damaging free radicals; decreasing platelet aggregation and so on.
As the metabolites containing sulphur amino acids, mammals have different abilities to synthesize taurine: The synthetic ability of rats and dogs is stronger, the synthetic ability of human and primate is lower, while that of kits and human infants is very low. Taurine in the infant mainly comes from the diet, so it is recommended to supplement the taurine in the baby's diet. Foods with a higher content of taurine include conch, clam, mussel, oyster, squid and other shellfish food, which chould be up to 500 ~ 900mg/100g in the table part; the content in fish is comparably different; the content in poultry and offal is also rich; the content in human milk is higher than cow milk; taurine is not found in eggs and vegetable food.
| [Medicinal effect]
- Liver-strengthening cholagogue function: The combination of taurine and cholic acid can increase biliary permeability and is related to bile backflow; this product can also reduce cholesterol levels in the liver and reduce the formation of cholesterol calculus.
- Anti-inflammatory and antipyretic effects: It can lower the body temperature by effects on the central 5-HT system or catecholamine system.
- Hypotensive effect: After injecting this product, it shows the effects including reducing blood pressure, slowing down heart rate, regulating vascular tension and so on.
- Cardiac and anti-arrhythmia action: This product can regulate the combination of Ca++ in cardiac myocytes and can reverse the adverse effects of Ca++ on the myocardium.
- Hypoglycemic effect: This product directly affects the insulin receptor of the liver and muscle cell membrane and has the effect of insulin-like hypoglycemic action.
- Other effects: loosening up skeletal muscle, reversing myotonia and fighting fatigue after exercise. Local application of this product can reduce the increased pressure in the eyeball caused by prostaglandin; there are still nutritional effects. Clinical use at acute hepatitis, chronic hepatitis, fatty liver, cholecystitis, etc.,as well as use in bronchitis, tonsillitis, ophthalmia and other infectious diseases. This product can be tried for cold, alcohol withdrawal symptoms, arthritis, myotonia, etc.
| [Preparation]
- Extract from the mollusk such as fish, shellfish and so on.
- After reacting to form sodium 2-hydroxyethanesulfonate at 70℃with ethylene oxide and sodium hydrogen sulfite as raw materials, this product can be obtained by further aminolysis and desalination [1].
- It can be obtained by reaction between ethylene imine and sulfurous acid [1].
- It can be obtained with nitroethylene and sodium bisulfite as raw materials[1]. CH2=CHNO2+NaHSO3→[1]
- It can be obtained preparing by sulfonation of sodium sulfite and aminolysis in liquid ammonia with sodium sulfite as the raw material [1].
- Ethanolamine is used as the raw material to react with hydrochloric acid to form chloroethylamine hydrochloride, which is reacted with sodium sulfite to produce sodium ethylamine sulfonate. This product can be obtained by desalination with dilute sulphuric acid [1].
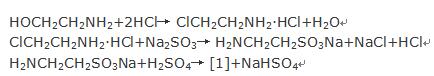
- Use aziridine as the raw material and react with sulfur dioxide and water to obtain this product [1].
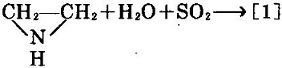
- Like 6, it can be obtained by using bromoethylamine hydrobromide as the raw material and reacting with sodium sulfite [1].
- Use 1, 2-dichloroethane as the raw material and react with sodium sulfite to produce chloroethanesulfonic acid sodium salt. React with ammonia under heating with pressure to form sodium amino ethyl sulfonate. Then it can be prepared by hydrochloric acid-acidification desalination [1].
- Like 9, we use hydroxyethanesulphonic acid as the raw material and react with ammonia under heating with pressure to obtain this product [1].
- We use 2,2-dimethyl thiazoles as the raw material, hydrogen peroxide or manganese dioxide as the oxidizing agent. This product can be obtained by oxidation under pressure [1], with acetone as by-product at the same time.
- It can be obtained by using 2-amino alcohol monoester as the raw material and reacting with sodium sulfite [1]. (H2NCH2CH2O)HSO3+Na2SO3→ [1]+Na2SO4
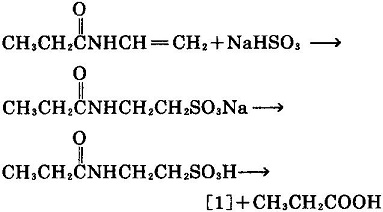
- N- vinyl propanamide is used as the raw material to react with sodium bisulfite to produce sodium 2- propane amino ethyl sulfonate. Then this product can be obtained by acidification desalination and hydrolysis.
| [References]
https://en.wikipedia.org/wiki/Taurine
https://pubchem.ncbi.nlm.nih.gov/compound/taurine#section=Top
|
|
|