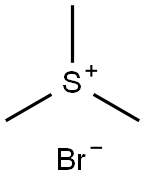
Trimethylsulfonium bromide synthesis
- Product Name:Trimethylsulfonium bromide
- CAS Number:3084-53-5
- Molecular formula:C3H9S.Br
- Molecular Weight:157.07
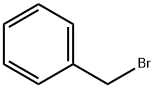
100-39-0
438 suppliers
$10.00/10 g

67-68-5
1286 suppliers
$15.00/100g

3084-53-5
210 suppliers
$14.30/5g
Yield:-
Reaction Conditions:
at 80; for 24 h;
Steps:
Firstly, (CH3)3SX(X = Cl, Br, I) were synthesized. For (CH3)3SI, equimolar amounts of CH3I and (CH3)2S were mixed in a round-bottomed flask andreacted at room temperature for 12 h. White crystals of (CH3)3SIwere formed and washed twice with diethyl ether [6]. For (CH3)3-SBr, a solution of benzyl bromide (2.38 mL, 20 mmol) and DMSO(10 mL) contained in a round-bottomed flask equipped with a condenserwas heated at 80 °C for 24 h. When the reaction mixturewas cooled to room temperature, trimethylsulfonium bromide(CH3)3SBr precipitated, which was then thoroughly washed withacetone and dried with P2O5 under vacuum [7]. (CH3)3SCl was synthesizedby mixing methyl chloroformate (0.025 mol, 2.37 g) withdimethyl sulfide (0.05 mol, 3.1 g) in a pressure bottle at 80 °C overnight[8]. The produced white crystals were washed with diethylether and dried with P2O5 under vacuum. Due to its hygroscopicnature, (CH3)3SCl was thereafter stored in an Argon-filled glovebox with O2 and H2O levels typically below 1 ppm.
References:
Kaltzoglou, Andreas;Elsenety, Mohamed M.;Koutselas, Ioannis;Kontos, Athanassios G.;Papadopoulos, Kyriakos;Psycharis, Vassilis;Raptopoulou, Catherine P.;Perganti, Dorothea;Stergiopoulos, Thomas;Falaras, Polycarpos [Polyhedron,2018,vol. 140,p. 67 - 73]
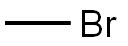
74-83-9
0 suppliers
$24.10/48624

75-18-3
408 suppliers
$18.10/8208330005

3084-53-5
210 suppliers
$14.30/5g

75-18-3
408 suppliers
$18.10/8208330005
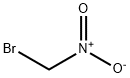
563-70-2
137 suppliers
$31.60/1g

3084-53-5
210 suppliers
$14.30/5g
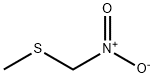
88096-62-2
0 suppliers
inquiry

75-18-3
408 suppliers
$18.10/8208330005
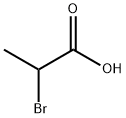
598-72-1
358 suppliers
$15.00/25g
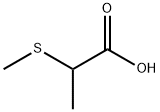
58809-73-7
22 suppliers
$35.00/200 μmol

3084-53-5
210 suppliers
$14.30/5g
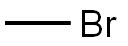
74-83-9
0 suppliers
$24.10/48624

1618-26-4
274 suppliers
$33.00/5mL

3084-53-5
210 suppliers
$14.30/5g